Abstract
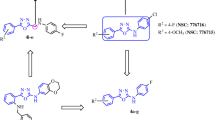
A novel series of tetrazole fused benzoxazole derivatives 9a–9j are synthesized, and their structures are characterized by 1H and 13C NMR, and mass spectra. The compounds 9a, 9b, 9g, 9h, and 9j demonstrate the highest activity. The compounds 9b and 9g exhibit good anticancer activity, in particular against MCF7, Hop62, and A-549 cell lines with the range of GI50 values from <0.1 to 4.56 μM. Molecular docking study is carried out for the compounds 9a–9j, according to which the compound 9b forms one hydrogen bond with THR766 with the highest docking score (–7.33). This indicates that 9b is effeciently binging to tubulin site.
Similar content being viewed by others
References
Sheng, C., Xu, H., Wang, W., Cao, Y., Dong, G., Wang, S., Che, X., Ji, H., Miao, Z., Yao, J., and Zhang, W., Eur. J. Med. Chem., 2010, vol. 45, p. 3531. doi 10.1016/j.ejmech.2010.03.007
Klimesova, V., Koci, J., Waisser, K., Kaustova, J., and Mollmann, U., Eur. J. Med. Chem., 2009, vol. 44, p. 2286. doi 10.1016/j.ejmech.2008.06.027
Liu, Y.K., Lou, D.J., Qian, J.Q., and Xu, Z.Y., J. Zhejiang. Univ. Sci., 2009, vol. 10, p. 472. doi 10.1631/jzus.B0820366
Prudhomme, M., Guyot, J., and Jeminet, G., J. Antibiot., 1986, vol. 39, p. 934. doi 10.7164/antibiotics.39.934
Srinivas, A., Vidyasagar, J., and Sarangapani, M., J. Chem. Pharm. Res., 2010, vol. 2, p.319.
Yildiz-Oren, I., Yalcin, I., Aki-Sener, E., and Ucarturk, N., Eur. J. Med. Chem., 2004, vol. 39, p. 291. doi 10.1016/j.ejmech.2003.11.014
Turan-Zitoni, G., Demirayak, S., Ozdemir, A., Kaplancikli, Z.A., and Yildiz, M.T., Eur. J. Med Chem., 2004, vol. 39, p. 267. doi 10.1016/j.ejmech.2003.11.001
Srinivas, A., Vidyasagar, J., Swathi, K., and Sarangapani, M., J. Chem. Pharm. Res., 2010, vol. 2, p.213.
Ueki, M., Shibata, K., and Taniguchi, M., J. Antibiot. 1998, vol. 51, p.883.
Ueki, M. and Taniguchi, M., J. Antibiot., 1997, vol. 50, p. 788
Diwakar, S.D., Bhagwat, S.S., Shingare, M.S., and Gill, C.H., Bioorg. Med. Chem. Lett., 2008, vol. 18, p. 4678. doi 10.1016/j.bmcl.2008.07.007
Bertinaria, M., Shaikh, M.A., and Buccellati, C., Chem. Med. Chem., 2012, vol. 7, p. 1647. doi 10.1002/cmdc.201200272
Yeung, K.S., Qiu, Z., Yang, Z. Bioorg. Med. Chem. Lett., 2013, vol. 23, p. 209. doi 10.1016/j.bmcl.2012.10.125
Romagnoli, R., Baraldi, P.G., and Salvador, M.K., J. Med. Chem., 2012, vol. 55, p. 475. doi 10.1021/jm2013979
Gundugola, A.S., Chandra, K.L., and Perchellet, E.M. Bioorg. Med. Chem. Lett.., 2010, vol. 20, p. 3920. doi 10.1016/j.bmcl.2010.05.012
Luo, Y.P., Gong, Q., Chen, Q., and Yang, G.F., Chin. J. Org. Chem., 2008, vol. 28, p. 1561.
Trecant, C., Dlubala, A., and George, P., Eur. J. Med. Chem., 2011, vol. 46, p. 4035. doi 10.1016/j.ejmech.2011.05.076
Scheffler, R.J., Colmer, S., Tynan, H., Demain, A.L., and Gullo, V.P., Appl. Microbiol. Biotechnol., 2013, vol. 97, p. 969. doi 10.1007/s00253-012-4609-8
Lee, P.Y., Chang, W.N., Lu, C.H., Lin, M.W., Cheng, B.C., Chien, C.C., Chang, C., and Chang, H.W., Antimicrob. Agents Chemother., 2003, vol. 51, p. 957. doi 10.1093/jac/dkg158
Balaraju, T., Kumar, A., Bal, C., Chattopadhyay, D., Jena, N., Bal, N.C., and Sharon, A., Struct. Chem., 2013, vol. 24, p. 1499.
Thiyagarajan, A., Salim, M.T., Balaraju, T., Bal, C., Baba, M., and Sharon, A., Bioorg. Med. Chem. Lett., 2012, vol. 22, p. 7742. doi 10.1016/j.bmcl.2012.09.072
Kasula, M., Balaraju, T., Toyama, M., Thiyagarajan, A., Bal, C., Baba, M., and Sharon, A., Chem. Med. Chem., 2013, vol. 8, p. 1673. doi 10.1002/cmdc.201300277
Genheden, S. and Ryde, U., Exp. Opinion Drug Disc., 2015, vol. 10, p. 449. doi 10.1517/17460441.2015.1032936
Li, J., Abel, R., Zhu, K., Cao, Y., Zhao, S., and Friesner, R.A., Proteins, 2011, vol. 79, p. 2794. doi 10.1002/prot.23106
Author information
Authors and Affiliations
Corresponding author
Additional information
The text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Ravikumar, P., Raolji, G.S.B., Venkata Sastry, K. et al. Design, Synthesis, and Anticancer Evaluation of Tetrazole-Fused Benzoxazole Derivatives as Tubulin Binding Agents. Russ J Gen Chem 88, 2183–2189 (2018). https://doi.org/10.1134/S1070363218100250
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363218100250