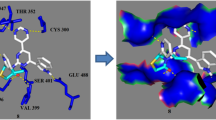
Abstract
1,2-Oxazole derivatives 1–6 were designed and evaluated computationally to calculate the physicochemical properties and the bioactivity score by Mol-inspiration, and were determined to possess very good activity score. 1,2-Oxazoles were then synthesized, characterized by FT-IR, 1H NMR and mass spectroscopy, and tested for antibacterial activity against the pathogens (Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, and Proteus mirabilis). The antibacterial therapeutic effect strongly supported the prior computational results. Four synthesized compounds 2, 4–6 demonstrated antibacterial potential higher than the standard drug Ciprofloxacin.
Similar content being viewed by others
References
Trojanowski, D., Skut, P., Hołówka, J., and Szafran, M.J., Postepy Hig. Med. Dosw., 2014, vol. 68, p. 701. doi 10.5604/17322693.1106890
Zhang, H.-Z., Zhao, Z.-L., and Zhou, C.-H., Eur. J. Med. Chem., 2018, vol. 144, p. 4442. doi 10.1016/j.ejmech.2017.12.044
Abhale, Y.K., Sasane, A.V., Chavan, A.P., Shekh S.H., Deshmukh, K.K., Bhansali, S., Nawale, L., Sarkar, D., and Mhaske, P.C., Eur. J. Med. Chem., 2017, vol. 132, p. 333. doi 10.1016/j.ejmech.2017.03.065
Moraski, G.C., Chang, M., Villegas-Estrada, A., Franzblau, SG., Möllmann, U., and Miller, M.J., Eur. J. Med. Chem., 2010, vol. 45, p. 1703. doi 10.1016/j.ejmech.2009.12.074
Zhongzhong, Y., Aiping, L., Mingzhi, H., Minhua, L., Hui, P., Lu, H., Haibo, Yi., Weidong, L., and Aixi, H., Eur. J. Med. Chem., 2018, vol. 149, p. 170. doi 10.1016/j.ejmech.2018.02.036
Chih-Hua, T., Chun-Kuang, L., Yeh-Long, C., Chin-Kai, T., Jar-Yu, L., and Jin-Ching, L., Eur. J. Med. Chem., 2018, vol. 143, p. 970. doi 10.1016/j.ejmech.2017.12.006
Tomi, I.H.R., Tomma, J.H., Al-Daraji, A.H.R., and Al-Dujaili, A.H., J. Saudi Chem. Soc., 2015, vol. 19 p. 392. doi 10.1016/j.jscs.2012.04.010
Padmavathi, V., Prema Kumari, C., Venkatesh, B.C., and Padmaja, A., Eur. J. Med. Chem., 2011, vol. 46, p. 5317. doi 10.1016/j.ejmech.2011.08.032
ShamsuzzamanMohd. Shaheen, K., Mahboob, A., Zishan, T., Anis, A., and Asad, U.K., Eur. J. Med. Chem., 2010, vol. 45, p. 1094. doi 10.1016/j.ejmech.2009.12.004
Seenaiah, D.P., Ramachandra, R.G., Mallikarjuna, R.A., Padmaja, V., and Padmavathi., K., Eur. J. Med. Chem., 2014, vol. 77, p. 1. doi 10.1016/j.ejmech.2014.02.050
Xin-Hua, L., Peng-Cheng, L., Jia-Yu, X., Bao-An, S., and Hai-Liang, Z., Eur. J. Med. Chem., 2009, vol. 44, p. 3930. doi 10.1016/j.ejmech.2009.04.019
Jie, Z., Jing, J., Yi, Z., Yuwen, Y., Xiaoguang, C., and Bailing, X., Eur. J. Med. Chem., 2013, vol. 68, p. 222. doi 10.1016/j.ejmech.2013.08.006
Chun-Liang, C., Fei-Lan, L., Chia-Chung, L., Tsung-Chih, C., Wen-Wei, C., Jih-Hwa, G., Ahmed, A.A.A., Chang, D.M., and Huang, H.S., Eur. J. Med. Chem., 2014, vol. 87, p. 30. doi 10.1016/j.ejmech.2014.09.016
Biersack, B., Effenberger, K., Knauer, S., Ocker, M., and Schobert, R., Eur. J. Med. Chem., 2010, vol. 45, p. 4890. doi 10.1016/j.ejmech.2010.07.061
Kumar, A., Ahmad, P., Maurya., R.A., Singh, A.B., and Srivastava, A.K., Eur. J. Med. Chem., 2009, vol. 44, p. 109. doi 10.1016/j.ejmech.2008.03.009
Mariappan, G., Saha, B.P., Sriparna Da. Deepak, K., and Haldar, P.K., J. Chem. Sci., 2011, vol. 123, p. 335. doi 10.1007/s12039-011-0079-2
Zhang, Y.X., Yan, J.F., Fan, L., Zhang, W.Y., Zhou, Z.W., Chen, X., Su, X.Y., Tang, X.M., Yang, D.C., and Yao, and Xue Xue Bao, 2009, vol. 44, p. 1244. doi 10.1007/978-3-540-93824-8_8811
Semenyuta, I., Kovalishyn, V., Tanchuk, V., Pilyo, S., Zyabrev, V., Blagodatnyy, V., Trokhimenko, O., Brovarets, V., and Metelytsia, L., Comput. Biol. Chem., 2016, vol. 65, p. 8. doi 10.1016/j.compbiolchem. 2016.09.012
Amira, S. Abd, El-All., Souad, A.O., Hanaa, M.F.R., Mohamed, M.A., Abeer, A.A., and Wafaa, H.A.-H., Med. Chem. Res., 2015, vol. 24, p. 4093. doi 10.1007/s00044-015-1460-3
Zhong, Z.J., Zhang, D.J., Peng, Z.G., Li, Y.H., Shan, G.Z., Zuo, L.M., Wu, L.T., Li, S.Y., Gao, R.M., and Li, Z.R., Eur. J. Med. Chem., 2013, vol. 69, p. 32. doi 10.1016/j.ejmech.2013.07.053
Donthamsetty, V.S., Shaik, S.B., Palampalli, U.M.D., Yerraguravagari, L., Adivireddy, P., and Venkatapuram, P., Med. Chem. Res., 2017, vol. 26, p. 1010. doi 10.1007/s00044-017-1801-5
Sutherland, R., Croydon, E.A.P., and Rolinson, G.N., Br. Med. J., 1970, vol. 21, p. 455. doi 10.1136/bmj.4.5733.455
Bhat, A.R., Arshad, M., Lee, E.J., Smritee, P., Inho, C., and Fareeda, A., Chem. Bio, 2013, vol. 10, p. 2267. doi 10.1002/cbdv.201300009
Arshad, M., Eur. J. Pharm. Med. Res., 2017, vol. 4 p. 511. https://doi.org/www.ejpmr.com/admin/assets/article_issue/1512459098.pdf.
Alodeani, E.A., Arshad, M., and Izhari, M.A., Asian Pac. J. Health Sci., 2015, vol. 2, p. 41. https://apjhs.com/pdf/8.pdf.
Alodeani, E.A., Arshad, M., and Izhari, M.A., Eur. J. Pharm. Med. Res., 2015, vol. 2, p. 324. https://doi.org/www.ejpmr.com/admin/assets/article_issue/1448880734.pdf.
Arshad, M., Bhat, A.R., Hoi, K.K, Inho, C., and Fareeda, A., Chin. Chem. Lett., 2017, vol. 28, p. 1559. doi 10.1016/j.cclet.2016.12.037
Arshad, M., Int. J. Pharm. Sci. Res., 2014, vol. 5, p. 1124. doi 10.13040/IJPSR.0975-8232.5(4).1000-13
Arshad, M., Int. J. Pharm. Sci. Res., 2014, vol. 5, p. 149. https://doi.org/www.ijpsr.info/docs/IJPSR14-05-04-001.
Author information
Authors and Affiliations
Corresponding author
Additional information
The text was submitted by the author in English.
Rights and permissions
About this article
Cite this article
Arshad, M. Synthesis, Characterization, and Antimicrobial Assessment of Some Computationally Bioactive 1,2-Oxazole Derivatives. Russ J Gen Chem 88, 1886–1891 (2018). https://doi.org/10.1134/S1070363218090207
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363218090207