Abstract
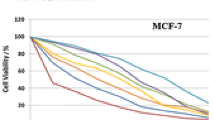
Five new cefradine (Cef) drug complexes were synthesized by 1 : 1 chemical reactions with Ca(II), Zn(II), Fe(III), Au(III), and Pd(II) ions in alkaline methanol/distilled water media. The general formula of Cef complexes can be presented as [M(Cef)Cl n ]·Cl y ·xH2O, where (M = CaII, ZnII, FeIII, AuIII, or PdII; n = 1, 2; y = 0,1; and x = 0, 3, 7, 14). Structures and physicochemical characteristics of complexes are studied by elemental analyses, IR, Raman and UV-Vis spectra, effective magnetic moments, X-ray powder diffraction (XRD), molar conductivity, SEM and TEM methods. Cef drug acts as a tridentate ligand toward the metal ions via oxygen atoms of the carbonyl β-lactam and carboxylate groups and nitrogen of the amino group. The effective magnetic moment of the paramagnetic Fe(III) complex in the solid state was assigned to the octahedral geometric structure. Antibacterial activity of the complexes were tested against some kinds of bacteria and fungi strains. Cytotoxicity of Au(III) complex was tested on human colon carcinoma (HCT-116) and hepatocellular carcinoma cells (HepG-2) using the MTT viability test. The results demonstrated the significant cytotoxic effect compared to the other complexes.
Similar content being viewed by others
References
Mahler, H.R. and Cordes, E.H., Biological Chemistry, New York: Harper and Rowe, 1966.
Iqbal, M.S., Ahmad, A.R., Sabir, M., and Asad, S.M., J. Pharm. Pharmacol., 1999, vol. 51, p. 371. doi 10.1211/0022357991772556
Anacona, J.R., Brito, L., and Peña, W., Synth. React. Inorg. Met.-Org. Nano-Met. Chem., 2012. vol. 42, p. 1278. doi org/10.1080/15533174.2012.680164
Anacona, J.R. and Brito, L. Lat., Am. J. Pharm., 2011, vol. 30(1), p. 172.
Shahzadi, S., Ali, S., Sharma, S.K., and Qanungo, K., J. Iran. Chem. Soc., 2010, vol. 7, p. 419. doi 10.1007/BF03246027
Sultana, N., Arayne, M.S., and Afzal, M., Pak. J. Pharm. Sci., 2003, vol. 16, p. 59.
Sultana, N., Arayne, M.S., and Afzal, M., Pak. J. Pharm. Sci., 2005, vol. 18, p. 36.
Anacona, J.R. and Acosta, F., J. Coord Chem., 59(6) (2006) 621. doi org/10.1080/00958 970500393208
Chohan, Z.H., and Jaffery, M.F., Met Based Drugs, 2000, vol. 7, p. 265. doi 10.1155/MBD.2000.265
Chaudhary, A., Phorc, A., Agarwalb, G.K., and Singh, R.V., Heterocyclic Commun, 2004, vol. 10, p. 393. doi org/10.1515/HC.2004.10.6.393
Auda, S.H., Mrestani, Y., Fetouh, M.I., and Neubert, R.H.H., Pharmazie, 2008, vol. 63, p. 555. doi 10.1691/ph.2008.8532
Anacona, J.R. and Rodriguez, A., Trans. Met. Chem., 2005, vol. 30, p. 897. doi 10.1007/s11243-005-6219-0
Al-Khodir, F.A.I. and Refat, M.S., Russ. J. Gen. Chem., 2015, vol. 85, p. 718. doi 10.1134/S1070363215030317
Al-Khodir, F.A.I. and Refat, M.S., J. Mol. Str., 2015, vol. 1094C, p. 22. doi 10.1016/j.molstruc.2015.03.063
Al-Khodir, F.A.I. and Refat, M.S., Russ. J. Gen. Chem., 2015, vol. 85, p. 1734. doi 10.1134/S1070363215070270
Al-Khodir, F.A.I. and Refat, M.S., J. Pharm. Innovation, 2015, vol. 10, p. 335. doi 10.1007/s12247-015-9230-9
Al-Khodir, F.A.I. and Refat, M.S., Russ. J. Gen. Chem., 2016, vol. 86, p. 708. doi 10.1134/S1070363216030324
Al-Khodir, F.A.I. and Refat, M.S., J. Mol. Struct., 2016, vol. 1119, p. 157. doi 10.1016/j.molstruc.2016.04.069
Mosmann, T., J. Immunol. Methods, 1983, vol. 65, p. 55. doi org/10.1016/0022-1759(83)90303-4
Gangadevi, V. and Muthumary, J., African J. Biotechnology, 2007, vol. 6, p. 1382. doi 10.5897/AJB2007.000-2194
Geary, W.J., Coord. Chem. Rev., 1971, vol. 7, p. 81. doi 10.1016/S0010-8545(00)80009-0
Refat, M.S., Spectrochim. Acta, Part A, 2007, vol. 68, p. 1393. doi 10.1016/j.saa.2006.12.078.
Nakamoto, K.., Infrared and Raman Spectra of Inorganic and Coordination Compounds, New York: Wiley, 1997.
Sharma, V.K. and Srivastava, S., Turk. J. Chem., 2006, vol. 30, p. 755.
Abdalrazaq, E.A., Buttrus, N.H., and Abd Al-Rahman, A.A., Asian J. Chem., 2010, vol. 22, p. 2179.
Tunney, J.M., Blake, A.J., Davies, E.S., Mcmater, J., Wilson, C., and Garner, C.D., Polyhedron, 2006, vol. 25, p. 591. doi 10.1016/j.poly.2005.09.002
Patterson, A.L., Phys. Rev., 1939, vol. 56, p. 978. doi org/10.1103/PhysRev.56.978
Malathy, M., and Rajavel, R., Smart Science, 2016, vol. 4, p. 95. doi 10.1080/23080477.2016.1196935
Author information
Authors and Affiliations
Corresponding author
Additional information
The text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Al-Khodir, F.A.I., Refat, M.S. Physicochemical, spectroscopic, and anti-tumor studies of cefradine complexes with Ca(II), Zn(II), Fe(III), Au(III), and Pd(II) ions. Russ J Gen Chem 87, 1087–1092 (2017). https://doi.org/10.1134/S1070363217050322
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363217050322