Abstract
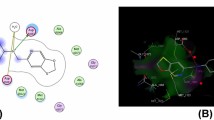
A series of novel {5-[4-hydroxy-3-(4-phenyl-2,3-dihydro-1H-benzo[b][1,4]diazepin-2-yl)benzyl]- benzofuran-2-yl}(phenyl)methanones (5a–5g) were prepared by the condensation of (E)-3-{5-[(2- benzoylbenzofuran-5-yl)methyl]-2-hydroxyphenyl}-1-phenylprop-2-en-1-one (chalcone) (4a) with various substituted o-phenylene diamines in the presence of oxalic acid as catalyst. The structures of all compounds were characterized by FTIR, 1H NMR, 13C NMR, and MS. The representative examples were screened in vitro for antimicrobial activity. Among all compounds 4g and 5g showed potent antibacterial activity, 4b and 5g showed good antifungal activity. The data was further compared with structure based investigations using docking studies with the crystal structure of adenosine-5'-(β,γ-imido)triphosphate (2ONJ) from Staphylococcus aureus for antibacterial activity and trypsin (1FY5) protein from Fusarium oxysporum for the antifungal activity. The score values estimated by genetic algorithm were found to have a good correlation with the experimental inhibitory potencies.
Similar content being viewed by others
References
Xue-Quan Wang, Lan-Xiang Liu, Yan Li, Cheng-Jun Sun, Wen Chen, Liang Li, Hong-Bin Zhang, Xiao-Dong Yang, Eur. J. Med. Chem., 2013, vol. 62, pp 111. DOI: 10.1016/j.ejmech.2012.12.040.
Yuhong, J.U. and Varma, R.S.J., J. Org. Chem., 2006, vol. 71, p. 135. DOI: 10.1021/jo051878h.
Sarma, B.K., Mishra, P.K., Singhai, P.K., and Singh, U.P., Mycobiology., 2007, vol. 35, p. 72. DOI: 10.4489/MYCO.2007.35.2.072.
Parikh, K.S. and Joshi, D.S., Med. Chem. Res., 2013, vol. 22, p. 3688. DOI: 10.1007/s004412-0369-3.
Dimmock, J.R., Ellas, D.W., Beazely, M.A., and Kandepu, N.M., Curr. Med. Chem., 1999, vol. 6(12), p. 1125.
De Meyer, N., Haemers, A., Mishra, L., Pandey, H.K., Peters, L.A., Vanden Berghe, D.A., and Vlietinck, A.J., J. Med. Chem., 1991, vol. 34, p. 736. DOI: 10.1021/jm00106a700.
Ducki, S., Forrest, R., Hadfield. J., Kendall, A., Lawerence, N.J., McGrown, A.T., and Rennison, D., Bioorg. Med. Chem. Lett., 1998 vol. 8, p. 1051. DOI: DOI: 10.1016/S0960-894X (98)00162-0.
Mei-Lin, Go., Liu Mei., Wilairat, P., Rosenthal, P.J., Saliba, K.J., and Kirk, K., Antimicrob Agents Chemother., 2004, vol. 48, p. 3241. DOI: 10.1128/AAC.48.9.3241-3245.2004.
Azam, A., Hayat, F., Mosely, E., Attar, S., and Vanzyl, R., Eur. J. Med. Chem., 2011, vol. 46, p. 1897. DOI: 10.1016/j.ejmech.2011.02.004.
Satyanarayana, M., Tiwari, P., Tripathi, B.K., Srivastava, A.K., and Pratap, R., Bioorg. Med. Chem., 2004, vol. 12, p. 883. DOI: 10.1016/j.bmc.2003.12.026.
Padhyee, S., Ahmad, A., Oswal, N., and Sarkar, F.H., J. Hematol. Oncol., 2009, vol. 2, p. 38. DOI: 10.1186/1756-8722-2-38.
Iwalewa, E.O., Oyedapo, O.A., Adewunmi, C.O., and Makunja, V.O., J. Biol. Sci., 2008, vol. 8, p. 131. DOI: 10.3923/jbs.2008.131.136.
Nam, N.H., Kim, Y., You, Y.J., Hong, D.H., Kim, H.M., and Ahn, B.Z., Eur. J. Med. Chem., 2003, vol. 38, p. 179. DOI: 10.1016/S0223-5234(02)01443-5.
Dimmock, J.R., Ellas, D.W., Beazely, M.A., and Kandepu, N.M., Curr. Med. Chem., 1999, vol. 6, p. 1125.
Khan, M.W., Alam, M.J., Rashid, M.A., and Chowdhury, R. A., Bioorganic & Medicinal Chemistry., 2005, vol. 13, p. 4796. DOI: 10.1016/j.bmc.2005.05.009.
Buu-Hoi, N.P., Bisagni, E., Royer, R., and Routier, C., J. Chem. Soc., 1957, p. 625. DOI: 10.1039/JR9570000625.
Buu-Hoi, N., Saint-Ruf, G., Loc, T.B., and Xuong, N.D., J. Chem. Soc., 1957, p. 2593. DOI: 10.1039/JR9570002593.
Benkli, K., Gundogdu-Karaburun, N., Karaburun, A.C., Ucucu, U., Demirayak, S., and Kiraz, N., Eur. J. Med. Chem., 2006, vol. 41, p. 651. DOI: 10.1016/j.ejmech.2005.12.013.
Baraldi, P.G., Romagnoli, R., Beria, I., Geroni, P.C., Mongelli, N., Bianchi, N., Mischiati, C., and Gambari, R., J. Med. Chem., 2000, vol. 43, p. 2675. DOI: 10.1021/jm9911229.
Chandrashekar. A., Eswarappa, B., Yadav, D.B., Venkatesh, K.B., Raghu, N., and Shivakumar, M.C., Med. Chem. Res., 2013, vol. 22, p. 78. DOI: 10.1007/s00044012-0017-y.
Middlemiss, D., Drew, G.M., Ross, B.C., Robertson, M.J., Scopes, D.C., Dowle, M. D., Hamblett, J., Cardwell, K., Clark, K. L., Coote, S., Eldred, C.D., Hamblett, J., Hilditch, A., Hirst, G.C., Jack, T., Montona, J., Panchal, T.A., Paton, S.M., Shah, P., Stuart, G., and Travers, A., Bio. Med. Chem. Lett., 1991, vol. 1, p. 711. DOI: 10.1016/S0960-894X(01)81053-2.
Leung, A.Y. and Foster, S.W., Encyclopedia of Common Natural Ingredients Used in Food, Drugs, and Cosmetics, New York: Wiley, 1996, vol. 35, p. 648.
Elvers, B., Hawkins, S., and Schulz, G., Optical Brighteners, Weinheim: VCH, 1999, vol. A18, p. 153, 5 ed.
Antoine, M., Barreu, M., Desconclois, J.F., Girard, P., and Picaut, G., Chem. Abstr., 1991, vol. 114, p. 14339.
Kusanur, R., Ghate, M., and Kulkurani, M., J. Chem. Sci., 2004, vol. 116, p. 265. DOI: 10.1007/BF02708277.
Basawaraj, R., Naubade, K., and Sangapure, S.S., Indian. J. Heterocycl. Chem., 2008, vol. 17, p. 217.
Grossi, G., Braccio, M.D., and Calcina, F., Eur. J. Med. Chem., 2002, vol. 37, p. 933. DOI: 10.1016/S0223-5234 (02)01400-9.
Kusanur, A.R., Ghate, M., and Kulkarni, V.M., J. Chem. Sci., 2004, vol. 11, p. 265. DOI: 10.1007/BF02708277.
Jauhari, P.K., Bhavani, A., Varalwar, S., Singhal, K., Raj, P., Med. Chem. Res., 2008, vol. 17, p. 412. DOI: 10.1007/s00044-007-9076-x.
Mahajan, A., Kumar, V., Mansour, N.R.N., Bickle, Q., and Chibale, K., Bioorg. Med. Chem. Lett., 2008, vol. 18, p. 2333. DOI: 10.1016/j.bmcl.2008.02.077.
Drabu, S. and Kumar, N., Asian. J. Chem., 2007, vol. 19, p. 5743.
Fred, E.D., Byung, H.L., Sandra, S.J., and Eileen, M.C., J. Med. Chem., 2003, vol. 46, p. 2057. DOI: 10.1021/jm020482k.
Gandhi, M. and Olyaei, A., J. Het. Chem., 2007, vol. 44, p. 323. DOI: 10.1002/jhet.5570440207.
Jadhav, S.B., Shasthri, R.A., Bagul, K.R., and Gaikwad, K.V., Indian. J. Het. Chem., 2009, vol. 18, p. 319. DOI: 10.9790/5736-09227175.
Marvel, C.S. and Tarkoy, N.J., Am. Chem. Soc., 1957, vol. 79, p. 6000. DOI: 10.1021/ja01579a041.
Thool, A.W. and Ghiya, B.J.J., Indian Chem. Soc., 1988, vol. 65, p. 522.
Aniket, P.S., Jaiprakashn, S., Nanasahe, B.B.D., Ajinkya, P.S., Paravani, S.W., and Devanand, B.S., J. Chil. Chem. Soc., 2013, vol. 58, p. N4. DOI: 10.4067/S0717-97072013000400064.
Bhavanarushi, S., Kanakaiah, V., Gandu, B., Gangagnirao, A., and Vatsala, R., Med. Chem. Res., 2014, vol. 23(1), p. 158. DOI: 10.1007/s00044-013-0623-3.
Patel, M.A., Bhila, V.G., Patel, N.H., Patel, A.K., and Brahmbhatt, D.I., Med. Chem. Res., 2012, vol. 21, p. 4381. DOI: 10.1007/s00044-012-9978-0.
Keche, A.P., Hatnapure, G.D., Tale, R.T., Rodge, A.H., Birajdar, S.S., and Kamble, V.M., Med. Chem. Res., 2013, vol. 22, p. 1480. DOI: 10.1007/s00044-012-0144-5.
Jones, G. and Willett, P., Glen, R.C., J. Mol. Biol., 1995, vol. 43, p. 245. DOI: 10.1016/S0022-2836(95) 80037-9.
Verdonk, M.L., Cole, J.C., Hartshorn, M.J., Murray, C.W., and Taylor, R.D., Struct. Funct. Bioinform., 2003, vol. 52, p. 609. DOI: 10.1002/prot.10465.
Trott, O. and Olson, A.J., J. Comp. Chem., 2010, vol. 31, p. 455. DOI: 10.1002/jcc.21334.
Dawson, R.J.P. and Locher, K.P., FEBS Lett., 2007, vol. 581, p. 935. DOI: 10.1016/j.febslet.2007.01.073.
Rypniewski, W.R., Ostergaard, P.R., Norregaard-Madsen, M., Dauter, M., and Wilson, K.S., Acta Crystallogr., Sect. D, 2001, vol. 57, p. 8. DOI: 10.1107/S0907444900014116.
Author information
Authors and Affiliations
Corresponding author
Additional information
The text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Shankar, B., Jalapathi, P., Ramesh, M. et al. Synthesis, antimicrobial evaluation, and docking studies of some novel benzofuran based analogues of chalcone and 1,4-benzodiazepine. Russ J Gen Chem 86, 1711–1721 (2016). https://doi.org/10.1134/S107036321607029X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107036321607029X