Abstract
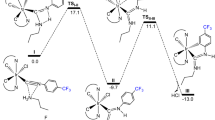
The electronic and steric features of acyclic aminohydrazinocarbene ligands in palladium(II) complexes are studied by the DFT method. It was found that most of the studied complexes prefer an amphi-2 conformation over all the other possible conformations. At the same time, in the ligand containing a hydrazide fragment more stable is a syn conformation. The basicity and nucleophilicity of the title ligands are much higher compared to the respective characteristics of the triphenylphosphine ligand and close to those of bis-(diisopropylamino)carbene.
Similar content being viewed by others
References
Correa, A., Nolan, S.P., and Cavallo, L., Top. Curr. Chem., 2011, vol. 302, p. 131. DOI: 10.1007/128-2010-118.
Boyarskiy, V.P., Luzyanin, K.V., and Kukushkin, V.Yu., Coord. Chem. Rev., 2012, vol. 256, p. 2029. DOI: 10.1016/j.ccr.2012.04.022.
Slaughter, L.M., ACS Catal., 2012, vol. 2, p. 1802. DOI: 10.1021/cs300300y.
Snead, D. R., Ghiviriga, I., Abboud, Kh. A., and Hong, S., Org. Lett., 2009, vol. 11, p. 3274. DOI: 10.1021/ol9013156.
Alder, R.W., Allen, P.R., Murray, M., and Orpen, A.G., Angew. Chem. Int. Ed., 1996, vol. 35, p. 1121. DOI: 10.1002/anie.199611211.
Alder, R.W., Blake, M.E., and Oliva, J.M., J. Phys. Chem. A, 1999, vol. 103, p. 11200.
Luzyanin, K.V., Tskhovrebov, A.G., Carias, M.C., Guedes da Silva, M.F.C., Pombeiro, A.J.L., and Kukushkin, V.Y., Organometallics, 2009, vol. 28, p. 6559. DOI: 10.1021/om900682v.
Miltsov, S., Karavan, V., Boyarsky, V., Gomez de Pedro, S., Alonso-Chamarro, J., and Puyol, M., Tetrahedron Lett., 2013, vol. 54, p. 1202. DOI: 10.1016/j.tetlet.2012.12.060.
Khaibulova, T.Sh., Boyarskaya, I.A., and Boyarskii, V.P., Russ. J. Org. Chem., 2013, vol. 49, p. 360. DOI: 10.1134/S1070428013030081.
Mikhailov, V.N., Savicheva, E.A., Sorokoumov, V.N., and Boyarskii, V.P., Russ. J. Org. Chem., 2013, vol. 49, p. 551. DOI: 10.1134/S107042801304009X.
Savicheva, E.A., Kurandina, D.V., Nikiforov, V.A., and Boyarskiy, V.P., Tetrahedron Lett., 2014, vol. 55, p. 2101. DOI: 10.1016/j.tetlet.2014.02.044.
Kinzhalov, M.A., Luzyanin, K.V., Boyarskiy, V.P., Haukka, M., and Kukushkin, V.Y., Organometallics, 2013, vol. 32, p. 5212. DOI: 10.1021/om4007592.
Valishina, E.A., Guedes da Silva, M.F.C., Kinzhalov, M.A., Timofeeva, S.A., Buslaeva, T.M., Haukka, M., Pombeiro, A.J.L., Boyarskiy, V.P., Kukushkin, V.Yu., and Luzyanin, K.V., J. Mol. Catal. A, 2014, vol. 395, p. 162. DOI: 10.1016/j.molcata.2014.08.018.
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery, J.A., Vreven, Jr., T., Kudin, K.N., Burant, J.C., Millam, J.M., Iyengar, S.S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G.A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J.E., Hratchian, H.P., Cross, J.B., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Ayala, P.Y., Morokuma, K., Voth, G.A., Salvador, P., Dannenberg, J.J., Zakrzewski, V.G., Dapprich, A.D., Daniels, M.C., Strain, O., Farkas, D.K., Malick, S., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Ortiz, J.V., Cui, Q., Baboul, A.G., Clifford, S., Cioslowski, J., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C. Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Gonzalez, C., and Pople, J.A., GAUSSIAN-03, Ver.5, Pittsburg, PA.: Gaussian, 2003.
Collins, M.S., Rosen, E.L., Lynch, V.M., and Bielawski, C.W., Organometallics, 2010, vol. 29, p. 3047. DOI: 10.1021/om1004226.
Bondi A., J. Phys. Chem., 1964, vol. 68, p. 441. DOI: 10.1021/j100785a001.
Lee, M.-T. and Hu, C.-H., Organometallics, 2004, vol. 23, p. 976. DOI: 10.1021/om0341451.
Becke, A.D., J. Chem. Phys., 1993, vol. 98, p. 5648. DOI: 10.1063/1.464913.
Stephen, P.J., Devlin, F.J., Ashvar, C.S., Chabalovski, C.F., and Frisch, M.J., Faraday Discuss., 1994, vol. 99, p. 103. DOI: 10.1039/FD9949900103.
Stephen, P.J., Devlin, F.J., Chabalovski, C.F., and Frisch, M.J., J. Phys. Chem., 1994, vol. 98, p. 11623. DOI: 10.1021/j100096a001.
Lee, C., Yang, W., and Parr, R.G., Phys. Rev. B, 1988, vol. 37, p. 785. DOI: 10.1103/PhysRevB.37.785.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © I.A. Boyarskaya, V.P. Boyarskii, 2015, published in Zhurnal Obshchei Khimii, 2015, Vol. 85, No. 4, pp. 654–658.
Rights and permissions
About this article
Cite this article
Boyarskaya, I.A., Boyarskii, V.P. Theoretical study of the structure of acyclic diaminocarbene ligands in Pd(II) complexes. Russ J Gen Chem 85, 894–898 (2015). https://doi.org/10.1134/S1070363215040222
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363215040222