Abstract
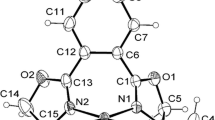
The reaction of anilines containing a 1,2,4-oxadiazole moiety with the bis(xylylisocyanide) Pd(II) complex leads to the formation of acyclic diaminocarbene complexes. The oxadiazole ring is not involved in the reaction. Composition and structure of the obtained complexes have been confirmed by means of mass spectrometry, NMR spectroscopy, and single-crystal X-ray diffraction analysis.




Similar content being viewed by others
REFERENCES
Vignolle, J., Cattoën, X., and Bourissou, D., Chem. Rev., 2009, vol. 109, no. 8, p. 3333. https://doi.org/10.1021/cr800549j
Slaughter, L.M., Adv. Synth. Catal., 2012, vol. 2, no. 8, p. 1802. https://doi.org/10.1021/cs300300y
Boyarskiy, V.P., Bokach, N.A., Luzyanin, K.V., and Kukushkin, V.Y.,Chem. Rev., 2015, vol. 115, no. 7, p. 2698. https://doi.org/10.1021/cr500380d
Tšupova, S., Rudolph, M., Rominger, F., and Hashmi, A.S.K.,Adv. Synth. Catal., 2016, vol. 358, no. 24, p. 3999. https://doi.org/10.1002/adsc.201600615
Kinzhalov, M.A. and Boyarskii, V.P., Russ. J. Gen. Chem., 2015, vol. 85, no. 10, p. 2313. https://doi.org/10.1134/S1070363215100175
Kinzhalov, M.A. and Luzyanin, K.V., Coord. Chem. Rev., 2019, vol. 399, p. 213014. https://doi.org/10.1016/j.ccr.2019.213014
Luzyanin, K.V., Tskhovrebov, A.G., Carias, M.C., Guedes da Silva, M.F.C., Pombeiro, A.J.L., and Kukushkin, V.Y., Organometallics, 2009, vol. 28, no. 22, p. 6559. https://doi.org/10.1021/om900682v
Kinzhalov, M.A., Luzyanin, K.V., Boyarskiy, V.P., Haukka, M., and Kukushkin, V.Y., Organometallics, 2013, vol. 32, no. 18, p. 5212. https://doi.org/10.1021/om4007592
Khaibulova, T.S., Boyarskaya, I.A., and Boyarskii, V.P., Russ. J. Org. Chem., 2013, vol. 49, no. 3, p. 360. https://doi.org/10.1134/S1070428013030081
Boyarskaya, D.V. and Boyarskii, V.P., Russ. J. Gen. Chem., 2016, vol. 86, no. 9, p. 2033. https://doi.org/10.1134/S1070363216090085
Mikhailov, V.N., Savicheva, E.A., Sorokoumov, V.N., and Boyarskii, V.P., Russ. J. Org. Chem., 2013, vol. 49, no. 4, p. 551. https://doi.org/10.1134/S107042801304009X
Miltsov, S., Karavan, V., Boyarsky, V., Gómez-de Pedro, S., Alonso-Chamarro, J., and Puyol, M., Tetrahedron Lett., 2013, vol. 54, no. 10, p. 1202. https://doi.org/10.1016/j.tetlet.2012.12.060
Mikhaylov, V.N., Sorokoumov, V.N., Korvinson, K.A., Novikov, A.S., and Balova, I.A., Organometallics, 2016, vol. 35, no. 11, p. 1684. https://doi.org/10.1021/acs.organomet.6b00144
Chay, R.S., Luzyanin, K.V., Kukushkin, V.Y., Guedes da Silva, M.F.C., and Pombeiro, A.J.L., Organometallics, 2012, vol. 31, no. 6, p. 2379. https://doi.org/10.1021/om300020j
Chay, R.S. and Luzyanin, K.V., Inorg. Chim. Acta, 2012, vol. 380, p. 322. https://doi.org/10.1016/j.ica.2011.09.047
Valishina, E.A., Guedes da Silva, M.F.C., Kinzhalov, M.A., Timofeeva, S.A., Buslaeva, T.M., Haukka, M., Pombeiro, A.J.L., Boyarskiy, V.P., Kukushkin, V.Y., and Luzyanin, K.V., J. Mol. Catal. (A), 2014, vol. 395, p. 162. https://doi.org/10.1016/j.molcata.2014.08.018
Timofeeva, S.A., Kinzhalov, M.A., Valishina, E.A., Luzyanin, K.V., Boyarskiy, V.P., Buslaeva, T.M., Haukka, M., and Kukushkin, V.Y., J. Catal., 2015, vol. 329, p. 449. https://doi.org/10.1016/j.jcat.2015.06.001
Savicheva, E.A., Kurandina, D.V., Nikiforov, V.A., and Boyarskiy, V.P., Tetrahedron Lett., 2014, vol. 55, no. 13, p. 2101. https://doi.org/10.1016/j.tetlet.2014.02.044
Boyarskii, V.P., Russ. J. Gen. Chem., 2017, vol. 87, no. 8, p. 1663. https://doi.org/10.1134/S1070363217080035
Ryabukhin, D.S., Sorokoumov, V.N., Savicheva, E.A., Boyarskiy, V.P., Balova, I.A., and Vasilyev, A.V., Tetrahedron Lett., 2013, vol. 54, no. 19, p. 2369. https://doi.org/10.1016/j.tetlet.2013.02.086
Mikhaylov, V.N., Sorokoumov, V.N., Liakhov, D.M., Tskhovrebov, A.G., and Balova, I.A., Catalysts, 2018, vol. 8, no. 4, p. 141. https://doi.org/10.3390/catal8040141
Valishina, E.A., Buslaeva, T.M., and Luzyanin, K.V., Russ. Chem. Bull., 2013, vol. 62, no. 6, p. 1361. https://doi.org/10.1007/s11172-013-0193-z
Singh, C., Prakasham, A.P., and Ghosh, P., ChemistrySelect., 2019, vol. 4, no. 1, p. 329. https://doi.org/10.1002/slct.201803292
Singh, C., Prakasham, A.P., Gangwar, M.K., Butcher, R.J., and Ghosh, P., ACS Omega, 2018, vol. 3, no. 2, p. 1740. https://doi.org/10.1021/acsomega.7b01974
Dhudshia, B. and Thadani, A.N., Chem. Commun., 2006, no. 6, p. 668. https://doi.org/10.1039/b516398f
Boyarskiy, V.P., Luzyanin, K.V., and Kukushkin, V.Y., Coord. Chem. Rev., 2012, vol. 256, no. 17, p. 2029. https://doi.org/10.1016/j.ccr.2012.04.022
Mikherdov, A.S., Baikov, S.V., Proskurina, I.K., Shetnev, A.A., and Kotov, A.D., Russ. J. Gen. Chem., 2019, vol. 89, no. 10, p. 2062. https://doi.org/10.1134/S1070363219100128
Serebryanskaya, T.V., Kinzhalov, M.A., Bakulev, V., Alekseev, G., Andreeva, A., Gushchin, P.V., Protas, A.V., Smirnov, A.S., Panikorovskii, T.L., Lippmann, P., Ott, I., Verbilo, C.M., Zuraev, A.V., Bunev, A.S., Boyarskiy, V.P., and Kasyanenko, N.A., New. J. Chem., 2020, vol. 44, no. 15, p. 5762. https://doi.org/10.1039/D0NJ00060D
Bertrand, B., Romanov, A.S., Brooks, M., Davis, J., Schmidt, C., Ott, I., O’Connell, M., and Bochmann, M., Dalton Trans., 2017, vol. 46, no. 45, p. 15875. https://doi.org/10.1039/C7DT03189K
Williams, M., Green, A.I., Fernandez-Cestau, J., Hughes, D.L., O’Connell, M.A., Searcey, M., Bertrand, B., and Bochmann, M., Dalton Trans., 2017, vol. 46, no. 39, p. 13408. https://doi.org/10.1039/C7DT02804K
Ivanov, D.M., Kinzhalov, M.A., Novikov, A.S., Ananyev, I.V., Romanova, A.A., Boyarskiy, V.P., Haukka, M., and Kukushkin, V.Y., Cryst. Growth. Des., 2017, vol. 17, no. 3, p. 1353. https://doi.org/10.1021/acs.cgd.6b01754
Kinzhalov, M.A., Baykov, S.V., Novikov, A.S., Haukka, M., and Boyarskiy, V.P., Z. Kristallogr. Cryst. Mater., 2019, vol. 234, no. 3, p. 155. https://doi.org/10.1515/zkri-2018-2100
Rassadin, V.A., Yakimanskiy, A.A., Eliseenkov, E.V., and Boyarskiy, V.P., Pharmaceuticals., 2015, vol. 61, p. 21. https://doi.org/10.1016/j.inoche.2015.08.008
Anisimova, T.B., Guedes da Silva, M.F.C., Kukushkin, V.Y., Pombeiro, A.J.L., and Luzyanin, K.V., Dalton Trans., 2014, vol. 43, no. 42, p. 15861. https://doi.org/10.1039/C4DT01917B
Tskhovrebov, A.G., Luzyanin, K.V., Dolgushin, F.M., Guedes da Silva, M.F.C., Pombeiro, A.J.L., and Kukushkin, V.Y., Organometallics, 2011, vol. 30, no. 12, p. 3362. https://doi.org/10.1021/om2002574
Kinzhalov, M.A., Timofeeva, S.A., Luzyanin, K.V., Boyarskiy, V.P., Yakimanskiy, A.A., Haukka, M., and Kukushkin, V.Y., Organometallics, 2016, vol. 35, no. 2, p. 218. https://doi.org/10.1021/acs.organomet.5b00936
Luzyanin, K.V., Pombeiro, A.J.L., Haukka, M., and Kukushkin, V.Y.,Organometallics, 2008, vol. 27, no. 20, p. 5379. https://doi.org/10.1021/om800517c
Mikherdov, A.S., Orekhova, Y.A., and Boyarskii, V.P., Russ. J. Gen. Chem., 2018, vol. 88, no. 10, p. 2119. https://doi.org/10.1134/S1070363218100158
Mikherdov, A.S., Tiuftiakov, N.Y., Polukeev, V.A., and Boyarskii, V.P., Russ. J. Gen. Chem., 2018, vol. 88, no. 4, p. 713. https://doi.org/10.1134/S1070363218040151
Gee, J.C., Fuller, B.A., Lockett, H.-M., Sedghi, G., Robertson, C.M., and Luzyanin, K.V., Chem. Commun., 2018, vol. 54, no. 68, p. 9450. https://doi.org/10.1039/C8CC04287J
Biernacki, K., Daśko, M., Ciupak, O., Kubiński, K., Rachon, J., and Demkowicz, S., Pharmaceuticals, 2020, vol. 13, no. 6, p. 111. https://doi.org/10.3390/ph13060111
Pace, A., Buscemi, S., Palumbo Piccionello, A., and Pibiri, I.,Adv. Heterocycl. Chem., 2015, vol. 116, p. 85. https://doi.org/10.1016/bs.aihch.2015.05.001
Welch, E.M., Barton, E.R., Zhuo, J., Tomizawa, Y., Friesen, W.J., Trifillis, P., Paushkin, S., Patel, M., Trotta, C.R., Hwang, S., Wilde, R.G., Karp, G., Takasugi, J., Chen, G., Jones, S., Ren, H., Moon, Y.-C., and Corson, D., Nature, 2007, vol. 447, no. 7140, p. 87. https://doi.org/10.1038/nature05756
Lanier, G., Sankholkar, K., and Aronow, W.S., Am. J. Ther., 2014, vol. 21, no. 5, p. 419. https://doi.org/10.1097/MJT.0b013e31824a0ed7
Hale, M., Wild, J., Reddy, J., Yamada, T., and Arjona Ferreira, J.C., Lancet Gastroenterol Hepatol., 2017, vol. 2, no. 8, p. 555. https://doi.org/10.1016/S2468-1253(17)30105-X
Krasavin, M., Shetnev, A., Sharonova, T., Baykov, S., Kalinin, S., Nocentini, A., Sharoyko, V., Poli, G., Tuccinardi, T., Presnukhina, S., Tennikova, T.B., and Supuran, C.T., Eur. J. Med. Chem., 2019, vol. 164, p. 92. https://doi.org/10.1016/j.ejmech.2018.12.049
Krasavin, M., Lukin, A., Vedekhina, T., Manicheva, O., Dogonadze, M., Vinogradova, T., Zabolotnykh, N., Rogacheva, E., Kraeva, L., Sharoyko, V., Tennikova, T.B. Dar’in, D., and Sokolovich, E., Eur. J. Med. Chem., 2019, vol. 166, p. 125. https://doi.org/10.1016/j.ejmech.2019.01.050
Atmaram Upare, A., Gadekar, P.K., Sivaramakrishnan, H., Naik, N., Khedkar, V.M., Sarkar, D., Choudhari, A., and Mohana Roopan, S., Bioorg. Chem., 2019, vol. 86, p. 507. https://doi.org/10.1016/j.bioorg.2019.01.054
Abdel hameid, M.K., Mohammed, M.R., Bioorg. Chem., 2019, vol. 86, p. 609. https://doi.org/10.1016/j.bioorg.2019.01.067
Caneschi, W., Enes, K.B., Carvalho de Mendonça, C., de Souza Fernandes, F., Miguel, F.B., da Silva Martins, J., and Costa Couri, M.R.,Eur. J. Med. Chem., 2019, vol. 165, p. 18. https://doi.org/10.1016/j.ejmech.2019.01.001
Salassa, G. and Terenzi, A., Int. J. Mol. Sci., 2019, vol. 20, no. 14, p. 3483. https://doi.org/10.3390/ijms20143483
Mayer, J.C.P., Sauer, A.C., Iglesias, B.A., Acunha, T.V., Back, D.F., Rodrigues, O.E.D., and Dornelles, L., J. Organomet. Chem., 2017, vol. 841, p. 1. https://doi.org/10.1016/j.jorganchem.2017.04.014.
Terenzi, A., Barone, G., Palumbo Piccionello, A., Giorgi, G., Guarcello, A., Portanova, P., Calvaruso, G., Buscemi, S., Vivona, N., and Pace, A., Dalton Trans., 2010, vol. 39, no. 38, p. 9140. https://doi.org/10.1039/c0dt00266f
Maftei, C.V., Fodor, E., Jones, P.G., Freytag, M., Franz, M.H., Kelter, G., Fiebig, H.H., Tamm, M., and Neda, I., Eur. J. Med. Chem., 2015, vol. 101, no. 1, p. 431. https://doi.org/10.1016/j.ejmech.2015.06.053
Kumari, S., Carmona, A.V., Tiwari, A.K., and Trippier, P.C.,J. Med. Chem., 2020. https://doi.org/10.1021/acs.jmedchem.0c00530
Bokach, N.A., Khripoun, A.V., Kukushkin, V.Y., Haukka, M., and Pombeiro, A.J.L., Inorg. Chem., 2003, vol. 42, no. 3, p. 896. https://doi.org/10.1021/ic026103v
Klapötke, T.M., Mayr, N., Stierstorfer, J., and Weyrauther, M.,Chem. Eur. J., 2014, vol. 20, no. 5, p. 1410. https://doi.org/10.1002/chem.201303825
Klingele, J., Kaase, D., Schmucker, M., and Meier, L., Eur. J. Inorg. Chem., 2013, vol, no. 28, p. 4931. https://doi.org/10.1002/ejic.201300511
Terenzi, A., Barone, G., Palumbo Piccionello, A., Giorgi, G., Guarcello, A., and Pace, A., Inorg. Chim. Acta, 2011, vol. 373, no. 1, p. 62. https://doi.org/10.1016/j.ica.2011.03.057
Bokach, N.A., Kukushkin, V.Y., Haukka, M., Pombeiro, A.J.L.,Eur. J. Inorg. Chem., 2005, vol. 2, no. 5, p. 845. https://doi.org/10.1002/ejic.200400580
Richardson, C. and Steel, P.J., Pharmaceuticals, 2007, vol. 10, no. 8, p. 884. https://doi.org/10.1016/j.inoche.2007.04.020
Tarasenko, M.V., Kofanov, E.R., Baikov, S.V., Krasovskaya, G.G., and Danilova, A.S., Russ. J. Org. Chem., 2017, vol. 53, no. 7, p. 1085. https://doi.org/10.1134/S1070428017070211
Dokla, E.M.E., Fang, C.-S., Abouzid, K.A.M., and Chen, C.S.,Eur. J. Med. Chem., 2019, vol. 182, p. 111607. https://doi.org/10.1016/j.ejmech.2019.111607
Maftei, C.V., Fodor, E., Jones, P.G., Franz, M.H., Kelter, G., Fiebig, H., and Neda, I., Beilstein J. Org. Chem., 2013, vol. 9, p. 2202. https://doi.org/10.3762/bjoc.9.259
Pathak, S.K., Nath, S., De, J., Pal, S.K., and Achalkumar, A.S.,New J. Chem., 2017, vol. 41, no. 18, p. 9908. https://doi.org/10.1039/C7NJ01766A
O’Daniel, P.I., Peng, Z., Pi, H., Testero S.A, Ding, D., Spink, E., Leemans, E., Boudreau, M.A., Yamaguchi, T., Schroeder, V.A., Wolter, W.R., Llarrull, L.I., Song, W., Lastochkin, E., Kumarasiri, M., Antunes, N.T., Espahbodi, M., and Lichtenwalter, K., J. Am. Chem. Soc., 2014, vol. 136, no. 9, p. 3664. https://doi.org/10.1021/ja500053x
Liu, J., Li, H., Chen, K.-X., Zuo, J.-P., Guo, Y.-W., Tang, W., and Li, X.-W., J. Med. Chem., 2018, vol. 61, no. 24, p. 11298. https://doi.org/10.1021/acs.jmedchem.8b01430
Sharonova, T., Pankrat’eva, V., Savko, P., Baykov, S., and Shetnev, A., Tetrahedron Lett., 2018, vol. 59, no. 29, p. 2824. https://doi.org/10.1016/j.tetlet.2018.06.019
Tarasenko, M., Duderin, N., Sharonova, T., Baykov, S., Shetnev, A., and Smirnov, A.V., Tetrahedron Lett., 2017, vol. 58, no. 37, p. 3672. https://doi.org/10.1016/j.tetlet.2017.08.020
Pankrat’eva, V.E., Sharonova, T.V., Tarasenko, M.V., Baikov, S.V., and Kofanov, E.R., Russ. J. Org. Chem., 2018, vol. 54, no. 8, p. 1250. https://doi.org/10.1134/S1070428018080213
Baykov, S., Sharonova, T., Shetnev, A., Rozhkov, S., Kalinin, S., and Smirnov, A.V., Tetrahedron, 2017, vol. 73, no. 7, p. 945. https://doi.org/10.1016/j.tet.2017.01.007
Shetnev, A., Osipyan, A., Baykov, S., Sapegin, A., Chirkova, Z., Korsakov, M., Petzer, A., Engelbrecht, I., and Petzer, J.P., Bioorg. Med. Chem. Lett., 2019, vol. 29, no. 1, p. 40. https://doi.org/10.1016/j.bmcl.2018.11.018
Shetnev, A., Baykov, S., Kalinin, S., Belova, A., Sharoyko, V., Rozhkov, A., Zelenkov, L., Tarasenko, M., Sadykov, E., Korsakov, M., and Krasavin, M., Int. J. Mol. Sci., 2019, vol. 20, no. 7, p. 1699. https://doi.org/10.3390/ijms20071699
Mikhaylov, V.N., Sorokoumov, V.N., Novikov, A.S., Melnik, M.V., Tskhovrebov, A.G., and Balova, I.A., J. Organomet. Chem., 2020, vol. 912, p. 121174. https://doi.org/10.1016/j.jorganchem.2020.121174
Kinzhalov, M.A., Boyarskiy, V.P., Luzyanin, K.V., Dolgushin, F.M., and Kukushkin, V.Y., Dalton Trans., 2013, vol. 42, no. 29, p. 10394. https://doi.org/10.1039/c3dt51335a
Kinzhalov, M.A., Luzyanin, K.V., Boyarskiy, V.P., Haukka, M., and Kukushkin, V.Y., Russ. Chem. Bull., 2013, vol. 62, no. 3, p. 758. https://doi.org/10.1007/s11172-013-0103-4
Conole, D., Beck, T.M., Jay-Smith, M., Tingle, M.D., Eason, C.T., Brimble, M.A., and Rennison, D., Bioorg. Med. Chem., 2014, vol. 22, no. 7, p. 2220. https://doi.org/10.1016/j.bmc.2014.02.013
Luzyanin, K.V., Guedes da Silva, M.F.C., Kukushkin, V.Y., and Pombeiro, A.J.L., Inorg. Chim. Acta, 2009, vol. 362, no. 3, p. 833. https://doi.org/10.1016/j.ica.2008.02.026
Kinzhalov, M.A., Kashina, M.V., Mikherdov, A.S., Mozheeva, E.A., Novikov, A.S., Smirnov, A.S., Ivanov, D.M., Kryukova, M.A., Ivanov, A.Y., Smirnov, S.N., Kukushkin, V.Y., and Luzyanin, K.V., Angew. Chem. Int. Ed., 2018, vol. 57, no. 39, p. 12785. https://doi.org/10.1002/anie.201807642
Srivastava, R.M., Pereira, M.C., Faustino, W.W.M., Coutinho, K., Dos Anjos, J.V., and De Melo, S.J., Monatsh. Chem., 2009, vol. 140, no. 11, p. 1319. https://doi.org/10.1007/s00706-009-0186-7.
Sheldrick, G.M., Acta Crystallogr. (A), 2015, vol. 71, no. 1, p. 3. https://doi.org/10.1107/S2053273314026370
Sheldrick, G.M., Acta Crystallogr. (C), 2015, vol. 71, no. 1, p. 3. https://doi.org/10.1107/S2053229614024218
Dolomanov, O.V., Bourhis, L.J., Gildea, R.J., Howard, J.A.K., and Puschmann, H., J. Appl. Crystallogr., 2009, vol. 42, no. 2, p. 339. https://doi.org/10.1107/S0021889808042726
Draghici, B., El-Gendy, B., and Katritzky, A., Synthesis, 2012, vol. 2012, no. 4, p. 547. https://doi.org/10.1055/s-0031-1289673
Funding
This study was financially supported by the Council for Grants of the President of Russian Federation (grant MK-1074.2020.3) and performed using the equipment of Resource Centers of St. Petersburg State University: “Magnetic Resonance Methods” and “X-ray Diffraction Methods.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Baikov, S.V., Trukhanova, Y.A., Tarasenko, M.V. et al. Synthesis and Study of the Structure of Palladium(II) Acyclic Diaminocarbene Complexes Containing a 1,2,4-Oxadiazole Moiety. Russ J Gen Chem 90, 1892–1900 (2020). https://doi.org/10.1134/S1070363220100126
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363220100126