Abstract
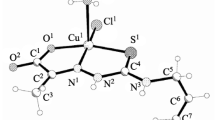
2-Formylpyridine 4-(2,6-dimethylphenyl)-(HL1), 4-(2,5-dimethylphenyl)-(HL2), 4-(3,4-dimethylphenyl)-(HL3), and 4-(2,4-dimethylphenyl)thiosemicarbazones (HL4) react with copper chloride and nitrate to form coordination compounds CuL1–4X·nH2O [X = Cl−, NO −3 ; n = 1, 2]. All compounds have a polynuclear structure. Azomethines HL1–4 act as the bridging monodeprotonated tridentate N,N,S-ligands. The thermolysis of the complexes includes the dehydration (70–90°C) and total thermal decomposition (350–520°C). The complexes synthesized exhibit a selective antimicrobial activity against a series of standard strains of Staphylococcus aureus and Escherichia coli in the concentration range of 0.009–37.5 μg ml−1.
Similar content being viewed by others
References
Mashkovskii, M.D., Lekarstvennye sredstva (Drugs), Moscow: Novaya Volna, 2008.
Zhungietu, G.I. and Granik, V.G., Osnovnya printsipy konstruirovaniya lekarstv (Basic Principles of the Drug Design), Chisinau: IPK Mold. Gos. Univ., 2000.
Gerbeleu, N.V., Arion, V.B., and Burges, J., Template Synthesis of Macrocyclic Compound, Weinheim: Wiley-VCH, 1999.
Samus’, N.M., Chumakov, Yu.M., Tapcov, V.I., Bocelli, G., Simonov, Yu.A., and Gulea, A.P., Zh. Obshch. Khim., 2009, vol. 79, no. 3, p. 439.
Chumakov, Yu.M., Tapcov, V.I., Jeannot, E., Bayrak, N.N., Bocelli, G., Poirier, D., Roy, J., and Gulea, A.P., Kristallografiya, 2008, vol. 53, no. 5, p. 833.
Gulea, A., Poirier, D., Roy, J., Stavila, V., Bulimestru, I., Tapcov, V., Birca, M., and Popovschi, L., J. Enzyme Inhib. Med. Chem., 2008, vol. 23, no. 6, p. 806.
Gulea, A.P., Spanu, S.N., Tapcov, V.I., and Poirier, D., Zh. Obshch. Khim., 2008, vol. 78, no. 5, p. 841.
Gulea, A.P., Prisakar, V.I., Tapcov, V.I., Buracheva, S.A., Spanu, S.N., and Bejenari, N.P., Khim.-Farm. Zh., 2008, vol. 42, no. 6, p. 41.
Arion, V.B., Gerbeleu, N.V., and Indrichan, K.M., Zh. Neorg. Khim., 1985, vol. 30, no. 1, p. 126.
Gulea, A.P., Prisakar, V.I., Tapcov, V.I., Buracheva, S.A., Spanu, S.N., Bejenari, N.P., Poirier, D., and Roy, J., Khim.-Farm. Zh., 2007, vol. 41, no. 11, p. 29.
Zelenin, K.N., Kuznetsova, O.B., Saminskaya, A.G., Alekseeva, V.V., Karimov, Z.T., Sivolodskii, E.P., Sofronov, G.A., Novikov, N.I., and Preobrazhenskaya, T.N., Khim.-Farm. Zh., 1994, vol. 31, no. 2, p. 34.
Ovsepyan, T.R., Gabrielyan, G.E., Simonyan, G.K., Arsenyan, F.G., Stepanyan, G.M., and Garibdzhanyan, B.T., Khim.-Farm. Zh., 2000, vol. 34, no. 5, p. 21.
Johari, R.B. and Sharma, R.C., J. Indian Chem. Soc., 1988, no. 11, p. 793.
Pershin, G.N., Metody eksperimental’noi khimioterapii (Methods of Experimental Chemotherapy), Moscow: Meditsina, 1971, p. 357.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.P. Gulea, K.S. Lozan-Tyrshu, V.I. Tsapkov, I.D. Korzha, V.F. Rudik, 2012, published in Zhurnal Obshchei Khimii, 2012, Vol. 82, No. 11, pp. 1880–1884.
Rights and permissions
About this article
Cite this article
Gulea, A.P., Lozan-Tyrshu, K.S., Tsapkov, V.I. et al. Coordination compounds of copper with 2-formylpyridine 4-(dimethylphenyl)thiosemicarbazones. Russ J Gen Chem 82, 1869–1872 (2012). https://doi.org/10.1134/S1070363212110242
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363212110242