Abstract
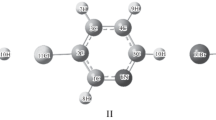
Equilibrium structural parameters, atomic charges, and quadrupole moments of the molecules of 2,4,6-tris(diazo)cyclohexane-1,3,5-trione, 3,6-bis(diazo)cyclohexane-1,2,4,5-tetraone and its anion, isomeric 3,6-bis(diazonium)cyclohexanediondioles and 4,4′-bis(diazonium)-1,1′-biphenyl in a vacuum and in dichloromethane were calculated by the quantum chemical method PBE0/cc-pVTZ using the polarizable continuum model (PCM).The p-quinoid dication is more favorable than o-quinoid dication by 20 kcal mol-1 in a vacuum and by 13 kcal mol−1 in dichloromethane.The norm of the quadrupole moment of the centrosymmetric molecules in a polarizable medium increases while that of the dications decreases. The quadrupole polarization of molecules and molecular ions by the solvent does not change their symmetry point group.
Similar content being viewed by others
References
D’yakonov, I.A., Alifaticheskie diazosoedineniya (Aliphatic Diazo Compounds), Lenungrad: Leningrad. Gos. Univ., 1958.
Semenov, S.G., Zh. Strukt. Khim., 1984, vol. 25, no. 6, p. 123.
Ansell, G.B., Hammond, P.R., Hering, S.V., and Corradini, P., Tetrahedron, 1969, vol. 25, no. 12, p. 2549.
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery, J.A., Jr., Vreven, T., Kudin, K.N., Burant, J.C., Millam, J.M., Iyengar, S.S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G.A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J.E., Hratchian, H.P., Cross, J.B., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Ayala, P.Y., Morokuma, K., Voth, G.A., Salvador, P., Dannenberg, J.J., Zakrzewski, V.G., Dapprich, S., Daniels, A.D., Strain, M.C., Farkas, O., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Ortiz, J.V., Cui, Q., Baboul, A.G., Clifford, S., Cioslowski, J., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Gonzalez, C., and Pople, J.A., GAUSSIAN-03, Revision B.05, Pittsburgh PA: Gaussian, 2003.
Tomasi, J. and Persico, M., Chem. Rev., 1994, vol. 94, p. 2027; Tomasi, J., Mennucci, B., and Cammi, R., Chem. Rev., 2006, vol. 105, p. 2999.
Jones, P.G., Ahrens, B., Höpfner, T., and Hopf, H., Acta Cryst., C, 1997, vol. 53, no. 6, p. 783.
Ansell, G.B., J. Chem. Soc., B, 1969, no. 6, p. 729.
Nikolaev, V.A., Shevchenko, V.V., Platz, M.S., and Khimich, N.N., Zh. Org. Khim., 2006, vol. 42, no. 6, p. 840; Shevchenko, V.V., Khimich, N.N., Platz, M.S., and Nikolaev, V.A., Zh. Org. Khim., 2006, vol. 42, no. 8, p. 1232.
Landau, L.D. and Lifshits, E.M., Teoriya polya (Field Theory), Moscow: Nauka, 1967.
Zülicke, L., Quantenchemie. Ein Lehrgang, Berlin: Deutscher Verlag der Wissenschaften, 1973, vol. 1.
Kirkwood, J.G., J. Chem. Phys., 1934, vol. 2, no. 7, p. 351.
Born, M., Z. Physik, 1920, vol. 1, no. 1, p. 45.
Dinur, U. and Hagler, A.T., J. Chem. Phys., 1989, vol. 91, no. 5, p. 2949; Dinur, U., Chem. Phys. Lett., 1990, vol. 166, no. 2, p. 211.
Semenov, S.G. and Mishina, N.N., Zh. Strukt. Khim., 2002, vol. 43, no. 5, p. 929.
Bader, R.F.W., Atoms in Molecules. A Quantum Theory, Oxford: Clarendon Press, 1990.
Glaser, R. and Horan, C.J., J. Org. Chem., 1995, vol. 60, no. 23, p. 7519.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © S.G. Semenov, M.V. Makarova, 2011, published in Zhurnal Obshchei Khimii, 2011, Vol. 81, No. 9, pp. 1465–1472.
Rights and permissions
About this article
Cite this article
Semenov, S.G., Makarova, M.V. Solvent induced quadrupole polarization of the molecules of diazo and diazocarbonyl compounds: Quantum-chemical investigation. Russ J Gen Chem 81, 1805–1811 (2011). https://doi.org/10.1134/S107036321109012X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107036321109012X