Abstract
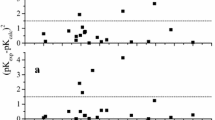
Dissociation constants of 15 carboxylic acids in water, methanol, and DMSO were calculated by empirical and quantum-chemical DFT methods. The maximal error of the empirical calculation was 1.4%. A comparative analysis of the results obtained was carried out.
Similar content being viewed by others
References
Kong, X., Zhou, T., Liu, Z., and Hider, R.C., J. Pharm. Sci., vol. 96, no. 10, p. 2777.
Box, K., Bevan, C., Comer, J., Hill, A., Allen, R., and Reynolds, D., Anal. Chem., 2003, vol. 75, no. 4, p. 883.
Jover, J., Bosque, R., and Sales, J., QSAR Comb. Sci., 2007, vol. 26, no. 3, p. 385.
Jover, J., Bosque, R., and Sales, J., QSAR Comb. Sci., 2008, vol. 27, no. 4, p. 563.
Jover, J., Bosque, R., and Sales, J., QSAR Comb. Sci., 2008, vol. 27, no. 10, p. 1179.
Ghasemi, J., Saaidpour, S., and Brown, S.D., J. Struct. Chem. THEOCHEM., 2007, vol. 805, nos. 1–3, p. 27.
Zhang, J., Kleinoeder, T., and Gasteiger, J., J. Chem. Inf. Model., 2006, vol. 46, no. 6, p. 2256.
Gieleciak, R. and Polanski, J., J. Chem. Inf. Model., 2007, vol. 47, no. 2, p. 547.
Ivanova, A.A., Baskin, I.I., Palyulin, V.A., and Zefirov, N.S., Dokl. Akad. Nauk, 2007, vol. 413, no. 6, p. 766.
Habibi-Yangjeh, A. and Danandeh-Jenagharad, M., Ind. J. Chem., Section B: Organic Chemistry Including Medicinal Chemistry, 2007, vol. 46, no. 3, p. 478.
Carabias-Martınez, R., Rodrıguez-Gonzalo, E., Domınguez-Alvarez, J., and Miranda-Cruz, E., Anal. Chim. Acta., 2007, vol. 584, no. 2, p. 410.
Jelfs, S., Ertl, P., and Selzer, P., J. Chem. Inf. Model., 2007, vol. 47, no. 2, p. 450.
Milletti, F., Storchi, L., Sforna, G., and Cruciani, G., J. Chem. Inf. Model., 2007, vol. 47, no. 6, p. 2172.
Popelier, P.L.A. and Smith, P.J., Eur. J. Med. Chem., 2006, vol. 41, no. 7, p. 862.
Shan, X., Qin, W., Zhou, Z., and Dai, Y., J. Chem. Eng. Data, 2008, vol. 53, no. 2, p. 331.
Hiromasa, K., Masamoto, A., and Kimito, F., J. Chem. Inf. Model., 2008, vol. 48, no. 3, p. 534.
Meloun, M. and Bordovska, S., Anal. Bioanal. Chem., 2007, vol. 389, no. 4, p. 1267.
Esteki, M., Hemmateenejad, B., Khayamian, T., and Mohajeri, A., Chem. Biol. Drug Design, 2007, vol. 70, no. 5, p. 413.
Vokin, A.I., Shulunova, A.M., Aksameentova, T.N., Bozhenkov, G.V., and Turchaninov, V.K., Zh. Obshch. Khim., 2006, vol. 76, no. 2, p. 311.
Stairs, R.A. and Buncel, E., Can. J. Chem., 2006, vol. 84, no. 11, p. 1580.
Yanchuk, N.I. and Grod, I.N., Zh. Obshch. Khim., 2006, vol. 76, no. 5, p. 757.
Reddy, S.R. and Manikyamba, P., Indian J. Chem. (A), 2006, vol. 45, no. 8, p. 1844.
Makitra, R.G., Midyana, G.G., and Pal’chikova, E.Ya., Zh. Org. Khim., 2005, vol. 41, no. 7, p. 967.
Lakhdar, S., Westermaier, M., Terrier, F., Goumont, R., Boubaker, T., Ofial, A.R., and Mayr, H., J. Org. Chem., 2006, vol. 71, no. 24, p. 9088.
Um, I.H., Hwang, S.J., and Buncel, E., J. Org. Chem., 2006, vol. 71, no. 3, p. 915.
Guven, A., Int. J. Mol. Sci., 2005, vol. 6, no. 11, p. 257.
Pavlat, P., Hlavac, J., and Bekarek, V., Chem. Listy, 2005, vol. 99, no. 8, p. 585.
Goumont, R., Magnier, E., Kizilian, E., and Terrier, F., J. Org. Chem., 2003, vol. 68, no. 17, p. 6566.
Lemp, E., Zanocco, A.L., and Lissi, E.A., Curr. Org. Chem., 2003, vol. 7, no. 9, p. 799.
Mchedlov-Petrosyan, N.O., Differentsirovanie sily organicheskikh kislot v istinnykh i organizovannykh rastvorakh (Differentiation of the Force of Organic Acids in True and Organized Solutions), Kharkov: Izd. Kharkov. Nat. Univer., 2004.
Adams, D.J., Dyson, P.J., and Tavener, S.J., Chemistry in Alternative Reaction Media, Guildfort: Wiley, 2004.
Reichardt, C., Solvents and Solvent Effects in Organic Chemistry, WeinHeim: WILEY-VCH Verlag GmbH, 2003.
Buncel, E., Stairs, R.A., and Wilson, H., Role of the Solvent in Chemical Reactions, Oxford: University Press, 2003.
Range, K., Riccardi, D., Cui, Q., Elstnerc, M., and York, D.M., Phys. Chem. Chem. Phys., 2005, vol. 7, no. 16, p. 3070.
Meot-Ner, M., Int. J. Mass Spectrometry, 2003, vol. 227, no. 3, p. 525.
Gross, K.C., Seybold, P., Peralta-Inga, Z., Murray, J.S., and Politzer, P., J. Org. Chem., 2001, vol. 66, no. 6919.
Fu, Y., Liu, L., Li, R.Q., Liu, R., and Guo, Q.X., J. Am. Chem. Soc., 2004, vol. 126, no. 3, p. 814.
Chipman, D.M., J. Phys. Chem. (A), 2002, vol. 106, no. 32, p. 7413.
Murowska, K. and Sadlej-Sosnowska, N., J. Phys. Chem. (A), 2005, vol. 109, no. 25, p. 5590.
Brown, T.N. and Mora-Diez, N., J. Phys. Chem. (B), 2006, vol. 110, no. 18, p. 9270.
Makowska, J., Makowski, M., and Chmurzynski, L., J. Phys. Chem. (A), 2004, vol. 108, no. 46, p. 10354.
Brown, T.N. and Mora-Diez, N., J. Phys. Chem. (B), 2006, vol. 110, no. 41, p. 20546.
Kelly, C.P., Cramer, C.J., and Truhlar, D.G., J. Phys. Chem. (A), 2006, vol. 110, no. 7, p. 2493.
Saracino, G.A., Improta, R., and Barone, V., Chem. Phys. Lett., 2003, vol. 373, nos. 3–4, p. 411.
Pal’m, V.A., Osnovy kolichestvennoi teorii khimicheskikh reaktsii (Foundations of the Quantitative Theory of Chemical Reactions), Leningrad: Khimiya, 1977.
Ulander, J. and Broo, A., Int. J. Quant. Chem., 2005, vol. 104, no. 6, p. 866.
Zevatskii, Yu.E. and Lysova, S.N., Zh. Prikl. Khim., 2006, vol. 79, no. 6, p. 978.
Zevatskii, Yu.E. and Vlasov, E.A., Zh. Obshch. Khim., 2007, vol. 77, no. 2, p. 260.
Zevatskii, Yu.E. and Samojlov, D.V., Zh. Prikl. Khim., 2007, vol. 80, no. 2, p. 230.
Zevatskii, Yu.E. and Samojlov, D.V., Zh. Org. Khim., 2008, vol. 44, no. 1, p. 59.
Bell, R.P, The Proton in Chemistry, New York: Cornell University Press, 1973.
Schrodinger, L.L.C. and Portland, O.R., Jaguar 7.0N, 2007, 1991–2003.
Miertus, S., Scrocco, E., and Tomasi, J., J. Chem. Phys., 1981, vol. 55, no. 1, p. 117.
Tomasi, J. and Persico, M., Chem. Rev., 1994, vol. 94, no. 7, p. 2027.
Cammi, R. and Tomasi, J., J. Comput. Chem., 1995, vol. 16, no. 12, p. 1449.
Salem, L., Electrons in Chemical Reactions: First Principles, New York, 1982.
Pliego, J.R., Chem. Phys. Letters, 2003, vol. 367, nos. 1–2, p. 145.
Zevatskii, Yu.E. and Samojlov, D.V., Zh. Org. Khim., 2008, vol. 44, no. 12, p. 1764.
Fialkov, Yu.Ya., Rastvoritel’ kak sredstvo upravleniya khimicheskim protsessom (Solvent as the Means of Controlling the Chemical Process), Leningrad: Khimiya, 1990.
Cherkasov, A.R., Galkin, V.I., and Cherkasov, R.A., Usp. Khim., 1996, vol. 65, no. 8, p. 695.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © Yu.E. Zevatskii, D.V. Samoilov, N.S. Panina, 2009, published in Zhurnal Obshchei Khimii, 2009, Vol. 79, No. 5, pp. 772–780.
Rights and permissions
About this article
Cite this article
Zevatskii, Y.E., Samoilov, D.V. & Panina, N.S. Calculations of dissociation constants of carboxylic acids by empirical and quantum-chemical DFT methods. Russ J Gen Chem 79, 944–952 (2009). https://doi.org/10.1134/S1070363209050132
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363209050132