Abstract
The Pt(II) acetylacetonate complex [(L)Pt(Acac)] based on aminophenyl-substituted 2-(2-thienyl)pyridine (L) was synthesized, its photophysical properties were studied and compared with these properties for a similar complex containing no amino group in the ligand. The structure of the complex was confirmed by X-ray diffraction (CIF file CCDC no. 2144689), 1H and 13C NMR spectroscopy, ESI mass spectrometry, and elemental analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The interest in the platinum(II) cyclometallated complexes is caused by the useful properties they exhibit, in particular luminescent properties [1, 2]. These complexes can be used to design OLEDs [3] and liquid crystalline materials [4]. Mention should also be made of their anticancer activity [5] and the use for the photogeneration of singlet oxygen [6].
This study addresses Pt(II) complexes based on 2‑(2-thienyl)pyridines with acetylacetone as the auxiliary ligand. These complexes are of interest for their photophysical characteristics [7, 8], applicability for the design of data storage devices [9], as reagents for photogeneration of singlet oxygen [10], and so on. Complexes of this type are also used to develop phosphorescent labels [11].
Previously [12], photophysical properties were studied for Pt(II) complexes of this type based on 5‑aryl-2-(2-thienyl)pyridines, which had luminescence lifetimes in the range of 17–21 µs and luminescence quantum yields of up to 28%. The subsequent functionalization of aromatic substituents in pyridine was used to introduce various functional groups able to bind to biological molecules and act as linkers, while the emission center was retained far from the biological substrate. In particular, this was shown earlier by introduction of a carboxyl group into the aryl substituent [11].
In this paper, we illustrate the possibility of generating a precursor of a phosphorescent label by introducing an amino group (which is of interest for the possibility to be converted to an isothiocyanate group [13]) into the aryl substituent of pyridine in order to obtain an amino-reactive luminescent dye. In this study, we show the possibility of preparing aminophenyl-containing Pt(II) complex based on 5-aryl-2-(2-thienyl)pyridine and report the results of studying the photophysical properties of this complex. It is noteworthy that no examples of synthesis of complexes of this type based on 2-(2-thienyl)pyridines containing an aromatic amino group were earlier reported in the literature.
EXPERIMENTAL
1H and 13C NMR spectra were recorded on a Bruker Avance-400 spectrometer (400 MHz) using SiMe4 as the internal standard. Electrospray ionization mass spectra were run on a MicrOTOF-Q II instrument (Bruker Daltonics, Bremen, Germany). Elemental analysis was performed on a PE 2400 II Perkin Elmer CHN analyzer. Macherey-Nagel, LOT045334009 silica gel (0.040–0.063 mm, 230–400 mesh) was used as the sorbent for column chro-matography. Silica gel-coated TLC Alu foils 91835 were used for thin layer chromatography. Starting 3‑(1-(2-thienyl)-6,7-dihydro-5H-cyclopenta[c]-pyridin-4-yl)aniline (ligand L) was obtained by a reported procedure [14]. All other compounds were commercial chemicals.
UV absorption spectra were measured on a Lambda 45 spectrophotometer (Perkin Elmer). Luminescence spectra were recorded on a Fluoromax-4 (Horiba) spectrofluorimeter using a NanoLED excitation source (without preliminary aeration). The absolute quantum yields were determined on a Fluoromax-4 (Horiba) spectrofluorimeter as described previously [15].
Synthesis of [(L)Pt(DMSO)(Cl)] (I). Thienylpyridine L (200 mg, 0.68 mmol) was dissolved in acetic acid (30 mL), and a solution of K2[PtCl4] (280 mg, 0.68 mmol) in water (5 mL) was added. The mixture was stirred at reflux under argon for 10 h. The bridged dimeric intermediate formed during the reaction was collected on a filter, washed with acetic acid, and dried in vacuo. Then DMSO (2 mL) was added, the reaction mixture was refluxed for 5 min and cooled down to room temperature, and water (15 mL) was added. The precipitate that formed was collected on a filter, washed with water, dried in vacuo, and dissolved in chloroform (10 mL). The insoluble impurities were filtered off. Evaporation of the mother liquor at a reduced pressure gave the target product, which was used in the next step without further purification. The yield was 200 mg (0.33 mmol, 49%). Tm > 250°C.
1H NMR (CDCl3, δ, ppm): 2.22 (m, 2H, CH2CH2CH2), 3.16 (m, 4H, CH2CH2CH2), 3.63 (s, 6H, CH3), 3.81 (br.s, 2H, NH2), 6.72 (m, 2H, H‑2′′,4′′), 6.80 (m, 1H, H-6′′), 7.22 (dd, J = 7.6 Hz, 1H, H-5′′), 7.48 (d, J = 5.2 Hz, 1H, H-5′), 7.73 (d, J = 5.2 Hz, 1H, H-4′), 9.20 (s, 1H, H-3).
Synthesis of [(L)Pt(Acac)] (II). A mixture of complex I (170 mg, 0.28 mmol) and sodium acetylacetonate monohydrate (400 mg, 2.8 mmol) was placed into acetone (50 mL). The resulting mixture was refluxed for 12 h. Acetone was removed at a reduced pressure, and the precipitate thus formed was purified by column chromatography (Rf = 0.35, dichloromethane (DCM)). The product was dried in vacuo. The yield was 95 mg (0.16 mmol, 57%). Tm > 250°C.
1H NMR (CDCl3; δ, ppm): 1.95 and 1.97 (both s, 3H, CH3), 2.20 (m, 2H, CH2-6), 3.08 (t, J = 7.4 Hz, 2H, CH2-7), 3.21 (t, J = 7.4 Hz, 2H, CH2-5), 3.78 (br.s, 2H, NH2), 5.46 (s, 1H, CH), 6.71 (m, 1H, H‑4′′), 6.75 (dd, J = 1.8 Hz, 1H, H-2′′), 7.25 (m, 2H, H-6′′,5′), 7.57 (d, J = 4.8 Hz, 1H, H-4′), 8.65 (m, 1H, H-3). 13C NMR (CDCl3; δ, ppm): 24.8, 26.7, 28.2, 31.7, 33.0, 100.0, 102.4, 114.5, 114.9, 118.7, 128.3, 129.6, 129.8, 133.3, 138.1, 138.5, 142.2, 144.7, 146.8, 154.9, 158.0, 183.7, 185.3. ESI-MS, m/z (Irel, %), found: 583.11 (1.89), 584.11 (0.58), 585.11 (77), 586.11 (100), 587.11 (85), 588.11 (22), 589.11 (22), 590.12 (5.4) (M + H)+; calcd.: 583.11 (1.84), 584.11 (0.49), 585.11 (77), 586.11 (100), 587.11 (87), 588.11 (24), 589.11 (23), 590.12 (5.7).
For C23H22N2O2SPt | |||
Anal. calcd., % | C, 47.18 | H, 3.79 | N, 4.78 |
Found, % | C, 46.84 | H, 3.83 | N, 4.55 |
The crystals of complex II suitable for X-ray diffraction were obtained by slow evaporation of its solution in CDCl3.
X-ray diffraction study of complex II was carried out using research equipment of the Center for Collective Use “Spectroscopy and Analysis of Organic Compounds” of the Postovsky Institute of Organic Synthesis, Ural Branch, Russian Academy of Sciences. The experiment was carried out for a plate-like brown crystal of 0.25 × 0.13 × 0.03 mm dimensions on an automated four-circle diffractometer with a Xcalibur 3 CCD array detector by a standard procedure (MoKα radiation, graphite monochromator, ω scan mode with a 1° step) at T = 295(2) K. An empirical absorption correction was applied. The structure was solved by the direct statistical method and refined by full-matrix least-squares method on F 2 in the anisotropic approximation for all non-hydrogen atoms. Hydrogen atoms were placed in the geometrically calculated positions and refined isotropically in the riding model. All calculations were carried out in the Olex software shell [16] using the SHELX program package [17].
The full set of X-ray diffraction parameters of II is deposited with the Cambridge Crystallographic Data Centre (CCDC no. 2144689; deposit@ccdc.cam.uk).
The main crystallographic parameters of compound II were as follows: orthorhombic crystal, space group Pbca, a = 7.2592(3) Å, b = 25.1219(18) Å, c = 26.115(2) Å, μ = 6.005 mm–1. For 2.85° < θ < 26.37° angles, 23 796 reflections were collected, of which 4851 reflections were unique (Rint = 0.0839), including 1943 reflections with I > 2σ(I). The final refinement parameters were R1 = 0.1114, wR2 = 0.1000 (for all reflections), R1 = 0.0395, wR2 = 0.0944 (for reflections with I > 2σ(I)) with GOOF = 1.002. The residual electron density peaks were 1.198/– 0.521 e Å–3.
RESULTS AND DISCUSSION
The initial ligand L was prepared by a previously described method [14] using the 1,2,4-triazine methodology [18]. Cyclometallated complex II was synthesized using the protocol described previously for related ligands [12]. The bridged dimeric complex obtained by the reaction of compound L and K2[PtCl4] was converted to the soluble dimethyl sulfoxide complex I by short-term heating in DMSO. The target acetylacetonate complex II was obtained by the reaction of I with sodium acetylacetonate; the product was purified by column chromatography (Scheme 1).
The presence of unprotected amino group did not affect the route of the above synthesis.

Scheme 1 .
The structure of the complex was confirmed by the data of 1H and 13C NMR spectra, ESI mass spectra, and elemental analysis. In addition, the crystals of chelate II suitable for X-ray diffraction were prepared and X-ray diffraction study was performed.
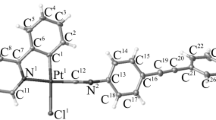
By the beginning of 2022, the Cambridge Structural Database contained information on 80 structures with a (pyridin-2-yl)thien-3-yl platinum moiety, including 20 structures with a 1,3-diketonate chelating group. Detailed considerations of the structure and properties of these complexes were reported [11, 19–21]. According to X-ray diffraction data, the compound crystallizes in the centrosymmetric space group of the orthorhombic system. The general view of the neutral complex molecule is shown in Fig. 1. The platinum atom has a distorted square geometry, usual for this type of complexes, with a planar arrangement of non-hydrogen atoms of the 1,3-diketonate and pyridylthienyl moieties (Fig. 1). The bond length asymmetry of the 1,3-diketonate chelate ring is rather well defined, contrary to expectations. The O(1) atom (O–C, 1.27(1) Å) is incorporated in the carbonyl moiety, while the O(2) atom (O–C, 1.34(1) Å) is a part of the enol group, which is also in good agreement with the Pt–O bond length asymmetry (2.055(7) and 1.994(8) Å for O(1) and O(2), respectively). Unfortunately, relatively low quality of X-ray diffraction data caused by small size of the crystal and low intensity of the diffraction pattern precludes more accurate estimation of the geometric characteristics of molecules.
The aza heterocyclic moiety of the complex is non-planar. In particular, the cyclopentylene moiety has an envelope conformation with deviation of the C(8) atom from the pyridine ring plane by 0.2 Å. The 3‑aminophenyl moiety is rotated relative to the pyridine ring plane by 50°. Possibly, shortened contacts are formed; the strict proof for the existence of these contacts requires further investigation.
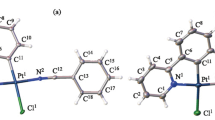
The molecules in the crystal form stacks (Figs. 2 and 3); neither specific metal–metal contacts nor substantially shortened interatomic π–π contacts are present in the crystal. Nevertheless, the relatively short distance from the central platinum atom to the pyridine ring plane of the neighboring molecule (3.54 Å) allows the formation of specific interactions within the molecule stacks. The cavities between the stacks (Fig. 3) are filled with the solvent. Due to the complexity of location of solvent atoms caused by great atomic thermal parameters, the solvent was included in the model as disordered water molecules in position 2 with occupancy factors of 0.5. This model gives satisfactory results regarding the convergence factor, thermal parameters, and geometry of interatomic interactions, but does not rule out the presence of other small solvent molecules (for example, MeOH or CH3CN) in the crystal cavities. The ultimate solution of this issue is possible only by conducting a low-temperature experiment with a higher-quality crystal.
We also studied the photophysical properties of new complex II. In a dichloromethane solution, II shows absorption and emission that are characteristic of previously described complexes based on related ligands [12] (Table 1, Figs. 4 and 5). However, the introduction of the amino group into the aromatic substituent of the ligand resulted in a significant decrease in the luminescence quantum yield and lifetime (the average lifetime is 2935.03 ns with a convergence factor of 1.25; the measurements were carried out at an excitation wavelength of 425 nm with an emission maximum at 575 nm). The detected trend coincides with that observed previously upon the introduction of the aromatic amino group into europium complexes based on 5-aryl-2,2'-bipyridines containing a DTTA residue in position C(6′) [22], despite different mechanisms of luminescence quenching.
Thus, we synthesized for the first time the Pt(II) acetylacetonate complex based on aminophenyl-substituted 2-(2-thienyl)pyridine; the aromatic amino group can be considered as a precursor of a linker for bioconjugation. The photophysical properties of the new complex were studied, and its crystal structure was determined by X-ray diffraction.
Change history
31 August 2022
An Erratum to this paper has been published: https://doi.org/10.1134/S1070328422340012
REFERENCES
Yoshida, M. and Kato, M., Coord. Chem. Rev., 2020, vol. 408, p. 213194.
Huo, S., Carroll, J., and Vezzu, D.A.K., Asian J. Org. Chem., 2015, vol. 4, no. 11, p. 1210.
Haque, A., Moll, H.E., Alenezi, K.M., et al., Materials, 2021, vol. 14, no. 15, p. 4236.
Spencer, M., Santoro, A., Freeman, G.R., et al., Dalton Trans., 2012, vol. 41, no. 47, p. 14244.
Cutillas, N., Yellol, G.S., de Haro, C., et al., Coord. Chem. Rev., 2013, vol. 257, nos. 19–20, p. 2784.
Djurovich, P.I., Murphy, D., Thompson, M.E., et al., Dalton Trans., 2007, no. 34, p. 3763.
Shafikov, M.Z., Kozhevnikov, D.N., Bodensteiner, M., et al., Inorg. Chem., 2016, vol. 55, no. 15, p. 7457.
Usuki, T., Uchida, H., Omoto, K., et al., Org. Chem., 2019, vol. 84, no. 17, p. 10749.
Poh, W.C., Au-Yeung, H.-L., Chan, A.K.W., et al., Chem. Asian J., 2021, vol. 16, no. 22, p. 3669.
Shafikov, M.Z., Suleymanova, A.F., Kutta, R.J., et al., J. Mater. Chem. C, 2021, vol. 9, no. 17, p. 5808.
Suleymanova, A.F., Kozhevnikov, D.N., and Prokhorov, A.M., Tetrahedron Lett., 2012, vol. 53, no. 39, p. 5293.
Kozhevnikov, D.N., Kozhevnikov, V.N., Ustinova, M.M., et al., Inorg. Chem., 2009, vol. 48, no. 9, p. 4179.
Hovinen, J. and Guy, P.M., Bioconjugate Chem., 2009, vol. 20, no. 3, p. 404.
Kopchuk, D.S., Khasanov, A.F., Kovalev, I.S., et al., Mendeleev Commun., 2013, vol. 23, no. 4, p. 209.
Porrés, L., Holland, A., Pålsson, L.-O., et al., J. Fluoresc., 2006, vol. 16, no. 2, p. 267.
CrysAlisPro. Agilent Technologies. Version 1.171.39.38a.
Sheldrick, G.M., Acta Crystallogr., Sect. A: Cryst. Adv., 2015, vol. 71, no. 1, p. 3.
Prokhorov, A.M. and Kozhevnikov, D.N., Chem. Heterocycl. Compd., 2012, vol. 48, no. 8, p. 1153. https://doi.org/10.1007/s10593-012-1117-9
Hao, Z., Zhang, K., Chen, K., et al., Chem. Asian J., 2020, vol. 15, no. 19, p. 3003.
Kumar, G.R. and Thilagar, P., Inorg. Chem., 2016, vol. 55, no. 23, p. 12220.
Venkatesan, K., Kouwer, P.H.J., Yagi, S., et al., J. Mater. Chem., 2008, vol. 18, no. 4, p. 400.
Prokhorov, A.M., Kozhevnikov, V.N., Kopchuk, D.S., et al., Tetrahedron, 2011, vol. 67, no. 3, p. 597.
ACKNOWLEDGMENTS
The authors are grateful to D.N. Kozhevnikov (Frishberg Institute of Applied Chemistry and Certification) for consultations during the study.
Funding
This study was supported by the Russian Science Foundation (grant no. 18-73-10119-P).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
The authors congratulate Academician V.I. Ovcharenko on the 70th birthday
Translated by Z. Svitanko
The original online version of this article was revised: Due to a retrospective Open Access order.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kopchuk, D.S., Slepukhin, P.A., Taniya, O.S. et al. Platinum(II) Acetylacetonate Complex Based on 5-(3-Aminophenyl)-2-(2-thienyl)pyridine: Synthesis, Crystal Structure, and Photophysical Properties. Russ J Coord Chem 48, 430–435 (2022). https://doi.org/10.1134/S1070328422070053
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328422070053