Abstract
The thermal sublimation of the known cage iron(II) complex (clathrochelate) gives thin films of this compound on various supports without violating its integrity as shown by electron spectroscopy. The spin state of the complex remains unchanged compared to the polycrystalline sample and solution. The first prototypes of molecular spintronic devices in the form of a vertical spin valve are prepared from the chosen iron(II) clathrochelate, and their electron transport properties are studied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
A possibility of using electron spin for information storage and processing has been of special interest for both fundamental science and diverse technological applications [1, 2] since the effect of giant magnetoresistance (GMR) was discovered by Fert [3] and Grünberg [4] who found the diffusion of spin-polarized charge carriers through the nonmagnetic layer contacting with the ferromagnetic layers. This discovery led to a revolution in the modern methods of information storage [5–7] and was a great stimulus for studying and developing devices of spin logics [8–10] and spintronics [1, 2, 7, 11–13] based on the influence of an external magnetic field on the electronic properties of matter. The further study of the GMR effect in the nonmagnetic metallic layer [1, 2, 14] made it possible to propose and accomplish in practice spintronic devices used for information reading and recording in modern computers [15–18] and as hypersensitive magnetic field sensors [19, 20], cells of energy-independent magnetoresistive operative memory [18, 21], and quantum computer elements [22, 23].
However, the range of potential applications of completely metallic spintronic devices is restricted by a short spin relaxation time (picoseconds) [24, 25] and impossibility to substantially change the character of conductance under external factors, for example, temperature or pressure [25–28]. An alternative to the nonmagnetic metallic layer in these devices can be organic semiconductors that have recently been considered actively [29–31]. They can efficiently affect spin injection [32, 33] and filtration [34–36] within an individual molecule [37], which forms a basis for the so-called “molecular spintronics” [38, 39].
Owing to the possibility of a direct interaction with the transport electron spin [40, 41], “molecular magnetics” are suitable for tasks of molecular spintronics [41, 42]. Molecular compounds with magnetic properties are traditionally attributed to molecular magnetics [25, 28]. Among them metal complexes with magnetic bistability are of special interest [43], since they allow one to monitor the dependence of the conducting properties of a spintronic device on the magnetic state of the central metal ion without serious structural rearrangements of the organic ligand [44]. Unfortunately, these compounds are often charged and have no high thermal stability [27] necessary for the formation of a related molecular layer by thermal sublimation traditionally used for manufacturing spintronic devices [25, 45–49].
Cage complexes (clathrochelates) of transition metals form one of the classes of compounds having a compensated charge and potentially suitable for the deposition on supports of various nature by thermal sublimation [50]. They are characterized by magnetic bistability in the form of a temperature-induced spin transition [51] and monomolecular magnetism [52–54].
In this work, we used one of the known representatives of this series, tris(dioximate) complex Co(GmCl2)3(BBu)2 (I) [50] in which the iron(II) ion is encapsulated by the cage ligand with the chlorine atoms and butyl groups as ribbed and apical substituents, respectively (Scheme 1), to prepare prototypes of molecular spintronic devices as a vertical spin valve based on the chosen unique class of “molecular magnetics” and to study their electron transport properties.

Scheme 1 .
EXPERIMENTAL
All procedures associated with the synthesis of the complex by a published method (Scheme 1 [50]) were carried out in air using commercially available n-butylboric acid, iron chloride FeCl2, organic solvents, and sorbents. Dichloroglyoxime and Fe(CH3CN)4Cl2 were synthesized using previously described procedures [55].
Analyses for carbon, nitrogen, and hydrogen were carried out on a Carlo Erba microanalyzer (model 1106). 1H and 13C NMR spectra were recorded in CD2Cl2 on a Bruker Avance 400 spectrometer with a working frequency of 400 MHz for 1H. The chemical shifts in the spectra were determined relative to the residual signal of the solvent.
Synthesis of complex I (Co(GmCl2)3(BBu)2). Dichloroglyoxime (6.28 g, 40 mmol) and n-butylboric acid (4.08 g, 22 mmol) were dissolved/suspended in anhydrous boiling nitromethane (70 mL) with stirring under argon, and Fe(CH3CN)4Cl2 (2.91 g, 10 mmol) was added gradually. The resulting reaction mixture was refluxed with stirring for 8 h, then the solvent was partially distilled off (30 mL), and the mixture was cooled to room temperature. A finely crystalline brown product was filtered off; washed with ethanol (20 mL, 2 portions), diethyl ether, and hexane; and dried in vacuo. The yield was 5.1 g (78%).
For C14H18B2N6O6Cl6Fe | |||
Anal. calcd., % | C, 25.59 | H, 2.74 | N, 12.80 |
Found, % | C, 25.69 | H, 2.75 | N, 12.84 |
1H NMR (CD2Cl2; 400 MHz; 290 K; δ, ppm): 0.72 (m, 4H, BCH2), 0.93 (t, 6H, CH3), 1.39 (m, 4H, CH2), 1.41 (m, 4H, CH2). 13C{1H} NMR (CD2Cl2; 100 MHz; 290 K; δ, ppm): 14.1 (s, CH3), 16.0 (br.s, BCH2), 25.6 (s, CH2), 25.8 (s, CH2), 130.5 (s, C=N).
Preparation of the films of complex I. The films were deposited on quartz supports 15 × 15 mm in size precleaned in air plasma for 60 s in a vaporization chamber in a box with an inert atmosphere by heating a finely crystalline powder of synthesized complex I to 350 K under a pressure of 10–5 Torr. The supports were preliminarily mounted in a special mask-holder that made it possible to simultaneously obtain several films under the same experimental conditions.
Electronic absorption spectra (UV and visible ranges) for the films on the supports were recorded in a range of 300–600 nm in a cryostat under argon on a Shimadzu i2600 spectrophotometer at 298 and 363 K. The spectra of the solution in dichloromethane were recorded in a range of 300–800 nm on a Cary 50 spectrophotometer at room temperature.
Scanning electron microscopy (SEM). The SEM images for bulky crystalline samples and films on the supports placed on a 25-mm aluminum stage and fixed by a conducting carbon ribbon were obtained in the secondary electron mode at an accelerating voltage of 5 kV on a Hitachi TM4000Plus desktop electron microscope. Elemental analysis of the surface was carried out using a Bruker Quantax 75 energy dispersive spectrometer connected to the microscope at an accelerating voltage of 15 kV.
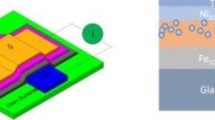
Preparation of the spintronic devices. Six devices of the “vertical spin valve” type, Ni(50)/I(50)/ V2O5(10)/Co(50) complex (Fig. 1), and two reference devices Ni(50)/V2O5(10)/Co(50), where the thickness of every layer in nm is indicated in parentheses, were prepared in two vaporization chambers in a box with an inert atmosphere. The metallic electrodes were deposited in one chamber, and the oxide layer and a layer of molecular complex I were deposited in another chamber. All layers were deposited under a pressure of 10–5 Torr from the corresponding powder using thermal sublimation on quartz supports 15 × 15 mm in size through masks of a necessary geometry. The deposited layer thickness was monitored using a quartz thickness gauge.
A layer of metallic nickel 50 nm thick was deposited on the support precleaned for 60 s in air plasma. Then a layer of complex I (50 nm thick) was deposited using the method described above, and this layer was covered with a thin (10 nm) V2O5 vanadium oxide layer. A layer of metallic cobalt 50 nm thick served as the upper electrode.
The prepared devices (Fig. 2) were encapsulated to measure current–voltage characteristics in air using a glass support 10 × 10 mm in size overlapping the active zone of the device but accessible for the electrode contacts.
Measurement of current–voltage characteristics. The current–voltage characteristics of the prepared devices were measured on a Keithley 2401 precision source-meter with the programmed specified voltage ranging from –10 to 10 V with an increment of 0.1 V at the established current maxima equal to –10 and 10 mA, respectively.
The resistance was measured at different applied external fields and a constant voltage of 100 mV. For a better contact, a conducting copper scotch was glued on the region of the ferromagnetic electrodes connected to the source-meter, and spring clamps were fastened to the scotch during measurements. An external magnetic field was generated by a BR-P100/40 electromagnet (Bairun Electric Co., China) switched to a QJ3003H laboratory power source (QJE, China) via a current polarity switcher. The magnetic induction at the surface of the measuring stage at the site of the arranged device prototype measured with a TU43205 laboratory teslameter (Russia) ranged from 0 to 50 mT.
The magnetoresistance was measured in two temperature modes (at room temperature and at ~80 K) and at two positions of the electromagnet: with the perpendicular and parallel magnetic field directions relative to the device plane. For low-temperature measurements, the device prototype together with the measuring stage and electromagnet was placed in a broad-neck Dewar vessel filled with liquid nitrogen.
RESULTS AND DISCUSSION
The chosen iron(II) tris(dioximate) clathrochelate (I) was synthesized using a described procedure [56] by the direct template reaction of dichloroglyoxime, n-butylboric acid, and the acetonitrile complex of anhydrous iron(II) chloride under harsh conditions (reflux in nitromethane) because of the low donor ability of dichloroglyoxime (Scheme 1). Rather readily volatile boric acid was partially distilled off during the reaction and, hence, a reflux condenser was used at the first stage. The formed water and HCl were azeotropically distilled off along with nitromethane thus shifting equilibrium toward the target product.
As mentioned previously [56], the iron(II) ion in complex I exists in the low-spin state, which was additionally confirmed by the electron spectroscopy data for a solution of the complex in dichloromethane. In fact, in the temperature range from 290 to 330 K the NMR spectra exhibit only the signals corresponding to the diamagnetic product, and the absorption spectra (Fig. 3b) at room temperature contain the metal-to-ligand charge-transfer bands with a maximum about 440 nm corresponding to the low-spin state of the iron(II) ion.
Films of the synthesized iron(II) clathrochelate were obtained on the surface of optically transparent (quartz) supports using for absorption spectra recording by heating a finely crystalline powder of the clathrochelate to 350 K under a pressure of 10–5 Torr. This method for film formation provided their homogeneity and equilibrium character, which is indicated by the SEM (Fig. 4) and electron spectroscopy data at different temperatures (Fig. 3a). The position and half-width of the metal-to-ligand charge-transfer bands in the absorption spectra of the film remained nearly unchanged with temperature thus indicating the low-spin state of the iron(II) ion in a range of 298–363 K, and no change in the base line, absorption intensity, and general contract of the absorption bands at the same temperature occurred upon repeated cooling/heating. The unchanged character of the absorption spectra and their resemblance with the corresponding spectrum of the solution in dichloromethane (Fig. 3b) indicate that the chosen complex retains its structure under the thermal sublimation conditions. In addition, according to the energy dispersive X-ray spectroscopy data, the elemental composition of the prepared film surface completely corresponded to that expected for complex I (Fig. 4b) and was identical to that in the bulky polycrystalline sample (Fig. 4c) except for the additionally found peak of silicon corresponding to the quartz support on which the films were immobilized for spectra recording.
(a) SEM image of the fragment of the film surface of complex I and the energy dispersive spectra of (b) this film and (c) the bulky polycrystalline sample of the initial complex. The appearance of the signals corresponding to silicon and a relative increase in the signals of oxygen in the spectra of the film are caused by silicon dioxide in the support material.
The possibility of the controlled formation of a homogeneous layer of the iron(II) clathrochelate on the support using thermal sublimation provides broad prospects for application as components of molecular spintronic devices. The simplest device is the so-called “vertical spin valve” [25, 57] in which two ferromagnetic electrodes prepared from materials with different coercitive forces are separated by the molecular compound layer [31, 32] with an additional thin layer of the insulator [25, 36, 58] providing the detection of the tunneling magnetoresistance [59]. Nickel [39, 45] and cobalt [25, 32, 35, 58, 60], which are appreciably different in coercitive force [61–64] and are popular in the area of molecular spintronics, were chosen as ferromagnetic electrodes for manufacturing vertical spin valve prototypes containing a layer of complex I as the corresponding molecular compound. This makes it possible to create a required configuration of the spintronic device with an opposite magnetization of the electrodes to provide spin filtration and, hence, magnetoresistance [25, 28, 59]. A layer of vanadium oxide V2O5, whose dielectric properties [65, 66] combined with high uniformity upon deposition [67] allows using it as a tunneling barrier in spintronic devices, served as the insulator.
Six spintronic devices of the “vertical spin valve” type (Fig. 1) of the Ni(50)/I(50)/V2O5(10)/Co(50) complex composition with the layer of the chosen iron(II) clathrochelate 50 nm thick and two reference devices of the Ni(50)/V2O5(10)/Co(50) composition (Fig. 2) without this layer were prepared by thermal sublimation in a box with an inert atmosphere. The current–voltage characteristics were measured for all prepared devices (Fig. 5). These characteristics represent lines obeying the Ohm law. Note a high reproducibility of the results of these measurements according to which the resistance for the main series of the spintronic devices is 264 ± 14 Ω, whereas the resistance of the reference devices is 65 ± 3 Ω. The character of the current–voltage curves indicates a high homogeneity of the layer formed by the thermal sublimation of complex I and the absence of short circuit or other defects capable of substantially affecting the electron transport properties of the prepared devices. Moreover, the encapsulation of the latter using a glass support made it possible to retain the current–voltage characteristics beyond the inert atmosphere of a glove box indicating that these devices can be used under real conditions (in air).
Magnetoresistance is the main characteristic of vertical spin valves. Magnetoresistance is a sharp change in the resistance of a spintronic device with a smooth change in the magnetic field corresponding to the transition from the parallel configuration (in which both ferromagnetic layers are similarly magnetized) to the antiparallel configuration (in which the external magnetic field application results in the remagnetization of the ferromagnetic electrode with a lower coercitive force). To measure the magnetoresistance of the prepared vertical spin valve prototypes, they were placed in the electromagnet field and their resistance was measured at a constant voltage difference on the contacts of the devices and with a smooth change in the current strength and polarity of the electromagnet. The experiments were carried out at both room temperature and liquid nitrogen temperature. As a result, a constant resistance coinciding with that obtained when measuring the current–voltage characteristics was found for each device regardless of the applied magnetic field induction and temperature. The same results were observed for the devices containing the iron(II) clathrochelate layer and their analogs in which the ferromagnetic electrodes are separated by a thin vanadium oxide tunneling barrier only. This fact indicates that the absence of magnetoresistance is related to the processes occurred in the tunneling barrier layer rather than to the fast spin relaxation in the clathrochelate. The replacement of the material of the layer or the fabrication of the devices with a thinner continuous tunneling barrier (e.g., based on partially oxidized aluminum [59, 68]) is the first step to the production of molecular spintronic devices based on transition metal clathrochelates, which are advantageous over other complexes by high thermal stability and wide possibilities of chemical modification for controlling the magnetic properties.
The possibility of preparing thin films on various supports by the thermal sublimation of transition metal clathrochelates or at least one representative of this series containing chlorine atoms and butyl groups in the ribbed and apical positions, respectively, and having a necessary thermal stability was first demonstrated in the present study. This made it possible to prepare the prototypes of molecular spintronic devices as a vertical spin valve with the electron transport characteristics demonstrating a high reproducibility. The very fast relaxation of the spin carriers in the tunneling oxide layer can be assumed from the absence of the expected magnetoresistance in the studied temperature range even for the reference devices having no layer of the chosen clathrochelate. The preparation of vertical spin valves with a thin tunneling barrier of alumina should decrease the observed relaxation effects and provide the fabrication of these devices with transition metal clathrochelates. In addition, the possibility of directed functionalization of their cage ligand combined with the encapsulation of various metal ions, which allows varying magnetic properties (including diverse manifestations of magnetic bistability), provides wide prospects for using this class of compounds in molecular spintronic devices with tunable operating characteristics.
REFERENCES
Wolf, S.A., Awschalom, D.D., Buhrman, R.A., et al., Science, 2001, vol. 294, p. 1488.
Žutič, I., Fabian, J., and Sarma, S.D., Rev. Mod. Phys., 2004, vol. 76, p. 323.
Baibich, M.N., Broto, J.M., Fert, A., et al., Phys. Rev. Lett., 1988, vol. 61, p. 2472.
Binasch, G., Grünberg, P., Saurenbach, F., and Zinn, W., Phys. Rev., 1989, vol. 39, p. 4828.
Slonczewski, J.C., J. Magn. Magn. Mater., 1996, vol. 159, р. L1.
Berger, L., Phys. Rev., 1996, vol. 54, p. 9353.
Bader, S.D. and Parkin, S.S.P., Annu. Rev. Condens. Matter Phys., 2010, vol. 1, p. 71.
Dery, H., Dalal, P., Cywiński, L., and Sham, L.J., Nature, 2007, vol. 447, p. 573.
Ney, A., Pampuch, C., Koch, R., and Ploog, K.H., Nature, 2003, vol. 425, p. 485.
Behin-Aein, B., Datta, D., Salahuddin, S., and Datta, S., Nat. Nanotechnol., 2010, vol. 5, p. 266.
Jedema, F.J., Filip, A.T., and van Wees, B.J., Nature, 2001, vol. 410, p. 345.
Schmidt, G., J. Phys. D: Appl. Phys., 2005, vol. 38, p. 323001.
Veev, J.P., Duan, C.G., Burton, J.D., et al., Nano Lett., 2009, vol. 9, p. 427.
Taguchi, K., Yokoyama, T., and Tanaka, Y., Phys. Rev., 2014, vol. 89. 085407.
Kim, Y., Yun, J.G., Park, S.H., et al., IEEE Trans. Electron Devices, 2012, vol. 59, p. 35.
Gajek, M., Nowak, J.J., Sun, J.Z., et al., Appl. Phys. Lett., 2012, vol. 100, p. 1.
Kawahara, T., Ito, K., Takemura, R., and Ohno, H., Microelectron. Reliab., 2012, vol. 52, p. 613.
Khvalkovskiy, A.V., Apalkov, D., Watts, S., et al., J. Phys. D: Appl. Phys., 2013, vol. 46, p. 074001.
Rao, C.N.R. and Cheetham, A.K., Science, 1996, vol. 272, p. 369.
Khvalkovskii, A.V. and Zvezdin, K.A., J. Magn. Magn. Mater., 2006, vol. 300, p. 270.
Bhatti, S., Sbiaa, R., Hirohata, A., et al., Mater. Today, 2017, vol. 20, p. 530.
Burkard, G., Engel, H.A., and Loss, D., Fortschritte der Phys., 2000, vol. 48, p. 965.
Clemente-Juan, J.M., Coronado, E., and Gaita-Ariñoa, A., Chem. Soc. Rev., 2012, vol. 41, p. 7464.
Naber, W.J.M., Faez, S., and van Der Wiel, W.G., J. Phys. D: Appl. Phys., 2007, vol. 40, p. R205.
Devkota, J., Geng, R., Subedi, R.C., and Nguyen, T.D., Adv. Funct. Mater., 2016, vol. 26, p. 3881.
Real, J.A., Gaspar, A.B., and Carmen Muñoz, M., Dalton Trans., 2005, vol. 12, p. 2062.
Coronado, E., Nature Rev. Mat., 2020, vol. 5, p. 87.
Forment-Aliaga, A. and Coronado, E., Chem. Rec., 2018, vol. 18, p. 737.
Dediua, S.B.V., Murgiaa, M., Matacottaa, F.C., et al., Hydrobiologia, 1980, vol. 71, p. 181.
Djeghloul, F., Ibrahim, F., Cantoni, M., et al., Sci. Rep., 2013, vol. 3, p. 1.
Xiong, Z.H., Wu, D., Vardeny, Z.V., and Shi, J., Nature, 2004, vol. 427, p. 821.
Wang, F.J., Yang, C.G., Vardeny, Z.V., and Li, X.G., Phys. Rev. B, 2007, vol. 75, p. 245324.
Barraud, C., Seneor, P., Mattana, R., et al., Nat. Phys., 2010, vol. 6, p. 615.
Torres-Cavanillas, R., Escorcia-Ariza, G., Brotons-Alcázar, I., et al., J. Am. Chem. Soc., 2020, vol. 142, p. 17572.
Droghetti, A., Thielen, P., Rungger, I., et al., Nat. Commun., 2016, vol. 7, p. 1.
Poggini, L., Cucinotta, G., Pradipto, A.M., et al., Adv. Mater. Interfaces, 2016, vol. 3, p. 1.
Schmaus, S., Bagrets, A., Nahas, Y., et al., Nat. Nanotechnol., 2011, vol. 6, p. 185.
Coronado, E. and Yamashita, M., Dalton Trans., 2016, vol. 45, p. 16553.
Yamada, R., Noguchi, M., and Tada, H., Appl. Phys. Lett., 2011, vol. 98, p. 053110.
Mercone, S., Perroni, C.A., Cataudella, V., et al., Phys. Rev. B, 2005, vol. 71, p. 1.
Bogani, L. and Wernsdorfer, W., Nanosci. Technol. A Collect. Rev. Nat. J., 2010, p. 194.
Urdampilleta, M., Nguyen, N.V., Cleuziou, J.P., et al., Int. J. Mol. Sci., 2011, vol. 12, p. 6656.
Novikov, V. and Nelyubina, Y., Russ. Chem. Rev., 2021, vol. 90, p. 703.
Bedoya-Pinto, A., Prima-García, H., Casanova, F., et al., Adv. Electron. Mater., 2015, vol. 1, p. 1.
Wang, K., Yang, Q., Duan, J., et al., Adv. Mater. Interfaces, 2019, vol. 6, p. 1.
Jiang, S.W., Chen, B.B., Wang, P., et al., Appl. Phys. Lett., 2014, vol. 104.
Ding, S., Tian, Y., Dong, H., et al., ACS Appl. Mater. Interfaces, 2019, vol. 11, p. 11654.
Bedoya-Pinto, A., Miralles, S.G., Vélez, S., et al., Adv. Funct. Mater., 2018, vol. 28, p. 1.
Xia, H., Zhang, S., Li, H., et al., Results Phys., 2021, vol. 22, p. 103963.
Voloshin, Y.Z., Kostromina, N.A., and Krämer, R.K., Clathrochelates: Synthesis, Structure and Properties, Elsevier Ltd., 2002.
Novikov, V.V., Ananyev, I.V., Pavlov, A.A., et al., J. Phys. Chem. Lett., 2014, vol. 5, p. 496.
Novikov, V.V., Pavlov, A.A., Nelyubina, Y.V., et al., J. Am. Chem. Soc., 2015, vol. 137, p. 9792.
Nehrkorn, J., Veber, S.L., Zhukas, L.A., et al., Inorg. Chem., 2018, vol. 57, p. 15330.
Aleshin, D.Y., Pavlov, A.A., Belova, S.A., et al., Russ. J. Inorg. Chem., 2019, vol. 64, p. 1532. https://doi.org/10.1134/S0036023619120027
Voloshin, Y.Z., Varzatskii, O.A., Novikov, V.V., et al., Eur. J. Inorg. Chem., 2010, p. 5401.
Voloshin, Y.Z., Varzatskii, O.A., Kron, T.E., et al., Inorg. Chem., 2000, vol. 39, p. 1907.
Cucinotta, G., Poggini, L., Pedrini, A., et al., Adv. Funct. Mater., 2017, vol. 27, p. 1703600.
Li, F., Li, T., Chen, F., and Zhang, F., Org. Electron., 2014, vol. 15, p. 1657.
Delprat, S., Galbiati, M., Tatay, S., et al., J. Phys. D: Appl. Phys., 2018, vol. 51, p. 473001.
Wang, F.J., Xiong, Z.H., Wu, D., et al., Synth. Met., 2005, vol. 155, p. 172.
Welch, R.H. and Speliotis, D.E., J. Appl. Phys., 1970, vol. 41, p. 1254.
Tanaka, T., Masuya, H., and Yazawa, K., IEEE Trans. Magn., 1985, vol. 21, p. 2090.
Miller, M.S., Stageberg, F.E., Chow, Y.M., et al., J. Appl. Phys., 1994, vol. 75, p. 5779.
Yamanaka, K., Takayama, T., Ogawa, Y., et al., J. Magn. Magn. Mater., 1995, vol. 145, p. 255.
Minagawa, M., Shinbo, K., Usuda, K., et al., Jpn. J. Appl. Phys., 2006, vol. 45, p. 1.
Schneider, K. and Maziarz, W., Sensors (Switzerland), 2018, vol. 18, p. 4177.
Kumar, A., Singh, P., Kulkarni, N., and Kaur, D., Thin Solid Films, 2008, vol. 516, p. 912.
Guo, L., Gu, X., Zhu, X., and Sun, X., Adv. Mater., 2019, vol. 31, p. 1.
ACKNOWLEDGMENTS
The authors are grateful to A.S. Belov (Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences) for the synthesis of the iron(II) clathrochelate using a published procedure. The elemental analysis of the synthesized complex was performed under the support of the Ministry of Science and Higher Education of the Russian Federation using the equipment of the Center for Molecular Composition Studies at the Nesmeyanov Institute of Organoelement Compounds (Russian Academy of Sciences).
Funding
This work was supported by the Russian Science Foundation, project no. 19-73-10194.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by E. Yablonskaya
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zlobin, I.S., Aisin, R.R. & Novikov, V.V. Iron(II) Clathrochelates in Molecular Spintronic Devices: A Vertical Spin Valve. Russ J Coord Chem 48, 33–40 (2022). https://doi.org/10.1134/S1070328422010080
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328422010080