Abstract
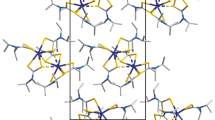
Crystalline bismuth(III) dithiocarbamato-chlorido complexes [Bi{S2CN(C3H7)2}Cl2] (I) and [Bi{S2CN(iso-C4H9)2}2Cl] (II) were prepared and comparatively studied by (13C, 15N) CP-MAS NMR spectroscopy, IR spectroscopy, and X-ray diffraction (CIF files CCDC nos. 1971976 and 1971975, respectively). The dithiocarbamate ligands are typically coordinated in the S,S'-iso- (I) or aniso- (II) bidentate-terminal mode. In each of the compounds, neighboring molecules are connected through one or two μ2-Cl– ligands into zigzag-like polymer chains, in which the central atom has sixfold coordination: [BiS2Cl4] (I) or [BiS4Cl2] (II). The binding unit in the polymer chains of I is a four-membered [Bi–(μ-Cl)2–Bi] metallocycle in the butterfly conformation (the dihedral angle is 140.51(3)°). Therefore, stronger binding of heteroleptic molecules in polymer chains leads to a considerably shorter interatomic Bi–Bi distances in I (4.0904(4) Å) than in II (4.8772(4) Å). The thermal behavior of heteroleptic bismuth(III) complexes was studied by simultaneous thermal analysis with recording of TG and DSC curves. Although the major product of thermal transformations of I and II is Bi2S3, the microprobe method also identified the presence of reduced bismuth and BiCl3.








Similar content being viewed by others
Notes
Some of the 13C CP-MAS NMR measurements were carried out on a Bruker Avance III 500 spectrometer (Bruker) operating at 125.76 MHz (B0 = 11.74 T) at the Umeå University, Umeå, Sweden.
REFERENCES
Koh, Y.W., Lai, C.S., Du, A.Y., et al., Chem. Mater., 2003, vol. 15, no. 24, p. 4544.
Ozturk, I.I., Banti, C.N., Kourkoumelis, N., et al., Polyhedron, 2014, vol. 67, p. 89.
Arda, M., Ozturk, I.I., Banti, C.N., et al., RSC Adv., 2016, vol. 6, no. 35, p. 29026.
Novikova, E.V., Ivanov, A.V., Egorova, I.V., et al., Russ. J. Coord. Chem., 2019, vol. 45, no. 10, p. 695. https://doi.org/10.1134/S1070328419100038
Jamaluddin, N.A., Baba, I., Halim, S.N.A., and Tiekink, E.R.T., Z. Kristallogr. NCS, 2015, vol. 230, no. 3, p. 239.
Battaglia, L.P. and Corradi, A.B., Dalton Trans., 1986, no. 8, p. 1513.
Raston, C.L., Rawbottom, G.L., and White, A.H., Dalton Trans., 1981, no. 6, p. 1352.
Adeyemi, J.O. and Onwudiwe, D.C., Molecules, 2020, vol. 25, no. 2, p. 305.
Raston, C.L., Rawbottom, G.L., and White, A.H., Dalton Trans., 1981, no. 6, p. 1366.
Raston, C.L., Rawbottom, G.L., and White, A.H., Dalton Trans., 1981, no. 6, p. 1379.
Raston, C.L., Rawbottom, G.L., and White, A.H., Dalton Trans., 1981, no. 6, p. 1372.
Bharadwaj, P.K., Lee, A.M., Skelton, B.W., et al., Aust. J. Chem., 1994, vol. 47, no. 2, p. 405.
Byr’ko, V.M. Ditiokarbamaty (Dithiocarbamates), Moscow: Nauka, 1984.
Hexem, J.G., Frey, M.H., and Opella, S.J., J. Chem. Phys., 1982, vol. 77, no. 7, p. 3847.
Harris, R.K., Jonsen, P., and Packer, K.J., Magn. Reson. Chem., 1985, vol. 23, no. 7, p. 565.
Pines, A., Gibby, M.G., and Waugh, J.S., J. Chem. Phys., 1972, vol. 56, no. 4, p. 1776.
Ratcliffe, C.I., Ripmeester, J.A., and Tse, J.S., Chem. Phys. Lett., 1983, vol. 99, no. 2, p. 177.
Sheldrick, G.M., Acta Crystallogr., Sect. A: Found. Adv., 2015, vol. 71, no. 1, p. 3.
Dolomanov, O.V., Bourhis, L.J., Gildea, R.J., et al., J. Appl. Crystallogr., 2009, vol. 42, no. 2, p. 339.
Ivanov, A.V., Gerasimenko, A.V., Egorova, I.V., et al., Russ. J. Coord. Chem., 2018, vol. 44, no. 8, p. 518. https://doi.org/10.1134/S1070328418080043
Yin, H.D., Li, F., and Wang, D., J. Coord. Chem., 2007, vol. 60, no. 11, p. 1133.
Brown, D.A., Glass, W.K., and Burke, M.A., Spectrochim. Acta, Part A, 1976, vol. 32, no. 1, p. 137.
Kellner, R., Nikolov, G.S., and Trendafilova, N., Inorg. Chim. Acta, 1984, vol. 84, no. 2, p. 233.
Kazitsyna, L.A. and Kupletskaya N.B., Primenenie UF-, IK-, YaMR- i mass-spektroskopii v organicheskoi khimii (Application of UV, IR, NMR, and Mass Spectroscopy in Organic Chemistry), Moscow: Izd. Mosk. Univ., 1979.
Lin, J.-C., Sharma, R.C., and Chang, Y.A., J. Phase Equilibr., 1996, vol. 17, no. 2, p. 132.
ACKNOWLEDGMENTS
X-ray diffraction studies were carried out using research equipment of the Molecular Structure Research Center of the Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences. Electron microscopy studies and recording of energy dispersive spectra were performed at the Analytical Center for Mineralogical and Geochemical Studies of the Institute of Geology and Nature Management, Far Eastern Branch, Russian Academy of Sciences.
The authors are grateful to the Knut and Alice Wallenberg Foundation (NMR for Life program), SciLifeLab Laboratory, and personally to Dr. Tobias Sparrman for help in conducting some 13C CP-MAS NMR experiments at the Umeå University, Umeå, Sweden.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by Z. Svitanko
Rights and permissions
About this article
Cite this article
Novikova, E.V., Isakovskaya, K.L., Antzutkin, O.N. et al. Heteroleptic Bismuth(III) Dithiocarbamato-Chlorido Complexes of [Bi(S2CNR2)Cl2] and [Bi(S2CNR2)2Cl] (R = C3H7, iso-C4H9): Preparation, 1D Polymer Structures, Heteronuclear (13C, 15N) CP MAS NMR, and Thermal Behavior. Russ J Coord Chem 47, 43–51 (2021). https://doi.org/10.1134/S1070328421010036
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328421010036