Abstract
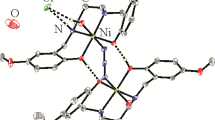
Three mononuclear different-ligand Zn(II) complexes, [Zn(CH3COO)2(PaoH)2] (I), [Zn(PaoH)2(DMSO)2][BF4]2 (II), and [Zn(NCS)2(PaoH)2] (III) (DMSO = dimethylsulfoxide) were prepared by the reaction of zinc acetate and tetrafluoroborate with pyridine-2-aldoxime (PaoH). The composition and structure of the complexes were confirmed by IR spectroscopy and X-ray diffraction. All compounds crystallize in the monoclinic system, compounds I and II have space group P21/n, while compound III has space group C2/c. In all compounds, the Zn coordination polyhedron is a distorted octahedron, which is formed by the N4O2 sets of donor atoms in I and II and by N6 in III. Complex I in the optimal concentration of 5–10 mg/L was found to stimulate the biosynthesis of standard (pH 4.7) amylases by the micromycete Aspergillus niger CNMN FD 06.
Similar content being viewed by others
References
Milios, C., Stamatatos, T., and Perlepes, S., Polyhedron, 2006, vol. 25, p. 134.
Polyzou, C., Efthymiou, C., Escuer, A., et al., Pure Appl. Chem., 2013, vol. 85, p. 315.
Zhang, S., Zhen, L., Xu, B., et al., Dalton Trans., 2010, vol. 39, p. 3563.
Zhou, C.-L., Wang, Z.-M., Wang, B.-W., and Gao, S., Dalton Trans., 2012, vol. 41, p. 13620.
Zheng, L., Zhang, S., Li, K., et al., J. Mol. Struct., 2010, vol. 984, p. 153.
Escuer, A., Vlahopoulou, G., and Mautner, F.A., Dalton Trans., 2011, vol. 40, p. 10109.
Konidaris, K., Katsoulakou, K., Kaplanis, M., et al., Dalton Trans., 2010, vol. 39, p. 4492.
Mancin, M., Tecilla, P., and Tonellato, U., Langmuir, 2000, vol. 16, p. 227.
Shih, T.-M., Skovira, J., and McDonough, J., Arch. Toxicol., 2009, vol. 83, p. 1083.
Coropceanu, E., Croitor, L., Siminel, A., and Fonari, M., Inorg. Chem. Commun., 2011, vol. 14, p. 1528.
Polyzou, C.D., Nikolaou, H., Papatriantafyllopoulou, C., et al., Dalton Trans., 2012, vol. 41, p. 13755.
Holyn'ska, M. and Korabik, M., Eur. J. Inorg. Chem., 2013, vol. 31, p. 5469.
Croitor, L., Coropceanu, E., Siminel, A., and Fonari, M., Polyhedron, 2013, vol. 60, p. 140.
Croitor, L., Coropceanu, E., Masunov, A., et al., Cryst. Growth Des., 2014, vol. 14, p. 3935.
Croitor, L., Grabco, D., Coropceanu, E., et al., Cryst-EngComm, 2015, vol. 17, p. 2450.
Croitor, L., Coropceanu, E., Petuhov, O., et al., Dalton Trans., 2015, vol. 44, p. 7896.
Papatriantafyllopoulou, C., Kostakis, G.E., Raptopoulou, C.P., et al., Inorg. Chim. Acta, 2009, vol. 362, p. 2361.
Torabi, A.A., Kian, R., Souldozi, A., and Welter, R., Z. Kristallogr.–New Cryst. Struct., 2005, vol. 220, p. 613.
Papatriantafyllopoulou, C., Raptopoulou, C., Terzis, A., et al., Z. Naturforsch., A: Phys. Sci., 2007, vol. 62, p. 1123.
Konidaris, K.F., Polyzou, C.D., Kostakis, G.E., et al., Dalton Trans., 2012, vol. 41, p. 2862.
Polyzou, C., Lada, Z., Terzis, A., et al., Polyhedron, 2014, vol. 79, p. 29.
Dermitzaki, D., Raptopoulou, C.P., Psycharis, V., et al., Dalton Trans., 2014, vol. 43, p. 14520.
Martinez, L., Gancheff, J.S., Hahn, F.E., et al., Spectrochim. Acta, Part A, 2013, vol. 105, p. 439.
Stamatatos, T., Katsoulakou, E., Terzis, A., et al., Polyhedron, 2009, vol. 28, p. 1638.
Stoumpos, C., Stamatatos, T., Sartzi, H., et al., Dalton Trans., 2009, vol. 6, p. 1004.
Papatriantafyllopoulou, C., Stamatatos, T., Efthymiou, C., et al., Inorg. Chem., 2010, vol. 49, p. 9743.
Papatriantafyllopoulou, C., Stamatatos, T., Wernsdorfer, W., et al., Inorg. Chem., 2010, vol. 49, p. 10486.
Papatriantafyllopoulou, C., Kostakis, G., Raptopoulou, C., et al., Inorg. Chim. Acta, 2009, vol. 362, p. 2361.
Sheldrick, G.M., Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, p. 112.
Deseatnic, A., Condruc, V., Bologa, O., et al., Patent MD2836, BIOPI, 2005, no.8.
Bershova, O.I. Mikroelementy i pochvennye mikroorganizmy (Nutrient Elements and Soil Mocroorganisms), Kiev: Naukova Dumka, 1967, p. 38.
Milios, C.J., Stamatatos, T.C., Kyritsis, P., et al., Eur. J. Inorg. Chem., 2004, p. 2885.
Milios, C.J., Kefalloniti, E., Raptopoulou, C.P., et al., Polyhedron, 2004, vol. 23, p. 83.
Milios, C.J., Kyritsis, P., Raptopoulou, C.P., et al., Dalton Trans., 2005, p. 501.
Stoumpos, C.C., Stamatatos, T.C., Psycharis, V., et al., Polyhedron, 2008, vol. 27, p. 3703.
Martinez, L., Gancheff, J.S., Hahn, F.E., et al., Spectrochim. Acta, Part A, 2013, vol. 105, p. 439.
Holynska, M., Z. Kristallogr., 2012, vol. 227, p. 831.
Liu, J., Acta Crystallogr, Sect. E: Struct. Rep. Online, 2014, vol. 70, p. 142.
Si, Y., Acta Crystallogr, Sect. E: Struct. Rep. Online, 2012, vol. 68, p. m554.
Shirvan, S.A. and Haydari Dezfuli, S., Acta Crystallogr, Sect. E: Struct. Rep. Online, 2012, vol. 68, p. m1080.
Ran, J. and Tong, Y.-P., Struct. Chem., 2011, vol. 22, p. 1113.
Konidaris, K.F., Bekiari, V., Katsoulakou, E., et al., Inorg. Chim. Acta, 2011, vol. 376, p. 470.
Zhuang, R.R., Jian, F.F., and Wang, K.F., J. Iran. Chem. Soc., 2011, vol. 8, p. 388.
Werner, M., Berner, J., and Jones, P.G., Acta Crystallogr., Sect. C; Cryst. Struct. Commun., 1996, vol. 52, p. 72.
Gracheva, I.M. Tekhnologiya fermentnykh preparatov (Preparation of Enzyme Specimens), Moscow: Agropromizdat, 1987, p. 36.
Gracheva, I.M., Grachev, Yu.P., et al., Laboratornyi praktikum po tekhnologii fermentnykh preparatov (Laboratory Works on the Preparation of Enzyme Specimens), Moscow: Legk. Pishch. Prom., 1982, p. 57.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.B. Coropceanu, L. Croitor, A.A. Ciloci, Zh.P. Tyurina, E.G. Dvornina, C.Z. Codreanu, M.S. Fonari, 2017, published in Koordinatsionnaya Khimiya, 2017, Vol. 43, No. 5, pp. 268–274.
Rights and permissions
About this article
Cite this article
Coropceanu, E.B., Croitor, L., Ciloci, A.A. et al. Synthesis and structure of mononuclear zinc complexes with pyridine-2-aldoxime. Russ J Coord Chem 43, 278–285 (2017). https://doi.org/10.1134/S1070328417050025
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328417050025