Abstract
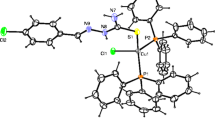
The crystal structures of (5-bromosalicylidenethiosemicarbazido)aquacopper(II) sulfato(5-bromosalicylidenethiosemicarbazido)aquacuprate(II) tetrahydrate (I), (5-nitrosalicylidenethiosemicarbazido)dimethylsulfoxide(methanol)copper(II) sulfate (II), and catena(μ-thiocyanato)(5-nitrosalicylidenethiosemicarbazido)copper(II) (III) were determined. In structure I, the independent part of the unit cell contains four water molecules of crystallization and the cation-anion complex [Cu(H2O)(L)][Cu(H2O)(L)(SO4)] containing two nonequivalent copper complexes (A and B). In these, the metal atom coordinates monodeprotonated 5-bromosalicylaldehyde thiosemicarbazone (HL) and water molecules, and in anion B also a sulfate ion. In complex ions A and B, sulfur, azomethine nitrogen, and phenolic oxygen of the salicylidene thiosemicarbazone fragment, and also water molecule form a distorted planar square around the metal atom. The axial vertex of the pyramid in anion B is occupied by the oxygen atom of the monodentate sulfate anion. In structure II, the independent part of the unit cell contains the (5-nitrosalicylidenethiosemicarbazido)dimethylsulfoxide(methanol)copper(II) complex cation and the sulfate anion. The coordination polyhedron of the central atom is a slightly distorted tetragonal pyramid whose base is formed by sulfur, azomethine nitrogen, phenolic oxygen of thiosemicarbazone, and dimethyl sulfoxide oxygen. The axial position is occupied by the methanol oxygen atom. In structure III, the copper atom coordinates 5-nitrosalicylaldehyde thiosemicarbazone and the thiocyanate ion, which combines complexes into infinite polymer chains along the [010] direction. The copper coordination polyhedron in III is a slightly distorted tetragonal pyramid whose base is formed by sulfur, azomethine nitrogen, phenolic oxygen of thiosemicarbazone, and nitrogen of thiocyanate ion, the axial vertex is occupied by sulfur of thiocyanate ion of the neighboring complex.
Similar content being viewed by others
References
Mashkovskii, M.D., Lekarstvennye sredstva (Pharmaceuticals), Minsk: Belarus’, 1987, vol. 2.
Zhungietu, G.I. and Granik, V.G., Osnovnye printsipy konstruirovaniya lekarstv (Main Principles of Drug Design), Chisinau: MoldGU, 2000.
Zelenin, K.N., Kuznetsova, O.B., Saminskaya, A.G., et al., Khim.-Farm. Zh., 1994, vol. 31, no. 2, p. 34.
Ovsepyan, T.R., Gabrielyan, G.E., Simonyan, G.K., et al., Khim.-Farm. Zh., 2000, vol. 34, no. 5, p. 21.
Prisakar’, V.I., Tsapkov, V.I., Buracheva, S.A., et al., Khim.-Farm. Zh., 2005, vol. 39, no. 5, p. 93.
Valdes-Martinez, J., Toscano, R.A., Zentella-Sehesa, A., et al., Polyhedron, 1996, vol. 15, p. 427.
Wang, D., Ebet, M., Schulzke, C., et al., Eur. J. Inorg. Chem., 2001, p. 935.
Labisbal, E., Haslow, K.D., Sousa-Pedrares, A., et al., Polyhedron, 2003, vol. 22, p. 2831.
De Meulenaer, J. and Tomp, H., Acta Crystallogr., 1965, vol. 19, p. 1014.
Sheldrick, G.M., SHELX-86. Program for the Solution and Refinement of Crystal Structure, Göttingen (Germany): Univ. of Göttingen, 1986.
Altomare, A., Burla, M.C., Camalli, M., et al., J. Appl. Crystallogr., 1999, vol. 32, p. 115.
Betteridge, P.W., Carruthers, J.R., Cooper, R.I., et al., J. Appl. Crystallogr., 2003, vol. 36, p. 1487.
Spek, A.L., J. Appl. Crystallogr., 2003, vol. 36, p. 7.
Bruno, I.J., Cole, J.C., Edginton, P.R., et al., Acta Crystallogr., Sect. B: Struct. Sci., 2002, vol. 58, p. 389.
Allen, F.H., Acta Crystallogr., Sect. B: Struct. Sci., 2002, vol. 58, p. 380.
Addison, A.W., Rao, J., Reedijk, J., et al., J. Chem. Soc., Dalton Trans., 1984, p. 1349.
West, D.X., Yang, Y., Klein, T.L., et al., Polyhedron, 1995, vol. 14, no. 12, p. 1681.
Labisbal, E., Haslow, K.D., Sousa-Pedrares, A., et al., Polyhedron, 2003, vol. 22, p. 2831.
Author information
Authors and Affiliations
Additional information
Original Russian Text © Yu.M. Chumakov, E. Janneau, N.P. Bezhenar’, V.I. Tsapkov, A.P. Gulya, 2008, published in Koordinatsionnaya Khimiya, 2008, Vol. 34, No. 1, pp. 46–54.
Rights and permissions
About this article
Cite this article
Chumakov, Y.M., Janneau, E., Bejenari, N.P. et al. Crystal structure of copper sulfate and thiocyanate complexes with 5-bromo-and 5-nitrosalicylaldehyde thiosemicarbazones. Russ J Coord Chem 34, 44–52 (2008). https://doi.org/10.1134/S1070328408010089
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328408010089