Abstract
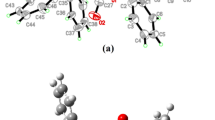
A complex [Zn(C8H7O3)2(H2O)2] (C8H8O3 is vanillin) has been synthesized and characterized by IR, elemental analysis, and X-ray diffraction single-crystal analysis. The crystals are monoclinic, space group C2/c, a = 22.236(8) Å, b = 10.594(2) Å, c = 7.8190(16) Å, α = 89.90(3)°, β = 106.87(4)°, γ = 89.99(3)°, V = 1762.6(8) Å3, Z = 4, F(000) = 832, S = 1.079, ρ c = 1.521g cm−3, R = 0.0221, R w = 0.0604, μ = 1.433 mm−1. The Zn2+ ion is six-coordinated with a distorted octahedron geometry. The complex forms a three-dimensional network through intermolecular hydrogen bonds. The thermal decomposition kinetics of the complex for the second stage was studied under non-isothermal conditions by the TG and DTG methods. The kinetic equation can be expressed as dα/dt = Ae−E/RT 2(1 − α)[1 − ln(1 − α)]1/2. The kinetic parameters (E, A), activation entropy ΔS ≠, and activation free-energy ΔG ≠ were also gained.
Similar content being viewed by others
References
Zhu, H.X., Deng, S.S., Zhang, H.B., et al., Chin. J. Fine Chemicals, 2004, vol. 21, p. 125.
Jin, S., Chin. J. Chemical Production and Technology. 2002, vol. 9, p. 41.
Burley, S.K., David, P.R., and Sweet, R.M., J. Mol. Biol., 1992, vol. 124, p. 133.
Naumann, C.F. and Prijs, B., J. Biochem., 1974, vol. 41, p. 209.
Sun, Y.G., Wei D.Z., Gao, E.J., and Ding, F., Chin. J. Acta Chim. Sinica, 2004, vol. 14, p. 136.
Liang, F.P., Chen, Z.L., Hu, R.X., et al., Chin. J. Inorg. Chem., 2001, vol. 5, p. 699.
Wang, L.Z., Wang, X.S., Li, Y.H., et al., Chin. J. Inorg. Chem., 2002, vol. 12, p. 1191.
Zhang, R.L., Liu, H.M., Zhao, J.Z., et al., Chin. J. Inorg. Chem., 2003, vol. 7, p. 722.
Zou, Y.N., Fan, Y.H., Bi, C.F., et al., Chin. J. Struct. Chem., 2006, vol. 3, p. 261.
Xiao, Z.J. and Liu, S.X., Chin. J. Struct. Chem., 2004, vol. 7, p. 798.
Ceng, M.H., Liang, H., and Shi, S.M., Chin. J. Struct. Chem., 2002, vol. 6, p. 654.
Li, D.G., Fu, W.Q., and You, X.Z., Chin. J. Struct. Chem., 2002, vol. 4, p. 431.
Sheldrick, G.M., SHELXTL-97, Program for Crystal Structure Refinement, Göttingen (Germany): Univ. of Göttingen, 1997.
Li, Y.Z., Thermal Analysis, Beijing: Qinghua University Press, 1987, p. 94.
Achar, B.N., Proceeding International Clay Conference, Book1, Jerusalem: 1966, p. 67.
Coats, A.W. and Redfern, J.P., Nature, 1964, vol. 201, p. 68.
Hu, R.Z. and Shi, Q.Z., Thermal Analysis Kinetics, Beijing: Science Press, 2001, p. 206.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Fan, Y.H., Zou, Y.N., Bi, C.F. et al. Synthesis, crystal structure, and kinetics of thermal decomposition of the Zn(II) complex with vanillin. Russ J Coord Chem 33, 570–575 (2007). https://doi.org/10.1134/S1070328407080040
Received:
Issue Date:
DOI: https://doi.org/10.1134/S1070328407080040