Abstract
Objective: To date, polychaetes remain a poorly studied class of invertebrate animals in terms of the features of functioning of their immune system and, in particular, the biodiversity of antimicrobial peptides (AMPs). AMPs also known as host defense peptides play a key role in host protection from various pathogens and regulation of the species composition of symbiotic microbes. A study of the biosynthesis of AMPs in polychaetes resulted in the discovery of the so-called BRICHOS domain in the structure of the precursor proteins of a number of such peptides. The conserved structure of this domain makes possible the bioinformatic search for AMP precursors in polychaete transcriptomes. In this work, we found and studied a novel BRICHOS-related AMP from the lugworm Arenicola marina, representing a previously undiscovered in polychaetes a structural family of defensin-like peptides stabilized by four disulfide bonds. Methods: The peptide, designated as AmBRI-44a and containing 44 amino acid residues, was obtained by heterologous expression in Escherichia coli. The peptide secondary structure was investigated by CD spectroscopy in water and dodecylphosphocholine (DPC) micelles. The minimum inhibitory concentrations (MICs) against a wide range of bacterial pathogens were assessed using the two-fold serial dilutions method. Cytotoxicity of AmBRI-44a was studied in vitro on human erythrocytes or adherent cell line HEK293T using the hemoglobin release assay or the MTT test, respectively. The AMBRI-44a potential target was discovered by successive daily subculturing of the AmBRI-44a resistant strain followed by whole-genome sequencing. Results and Discussion: According to CD data, AmBRI-44a is a predominantly β-structured peptide. AmBRI-44a was shown to have a specific activity against a narrow spectrum of Gram-positive bacteria and pronounced cytotoxic effects on the eukaryotic cell line HEK293T. The proposed mechanism of the antibacterial action of this peptide is associated with the inhibition of bacterial cell wall biosynthesis, as indicated by the genetic and phenotypic analysis of selected AmBRI-44a-resistant bacteria Bacillus licheniformis B-511. Conclusions: The resulting data allow us to consider the discovered peptide AmBRI-44a as a candidate compound for the development of an antibiotic agent that could potentially be effective in the treatment of infectious diseases mediated by multidrug-resistant Gram-positive bacteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Antimicrobial peptides (AMPs) are key molecular factors of the innate immune system of most multicellular species, including humans [1]. AMPs are synthesized on the ribosome and perform a protective function in animals, plants, and fungi and also play a communicative role in bacteria. In the process of evolution over hundreds of millions of years, marine multicellular organisms living in the salt water of all seas and oceans, from the Arctic to the Antarctic, have constantly improved and successfully formed reliable protective mechanisms of innate immunity [2]. At the same time, compared to terrestrial species, marine animals have been scarcely studied in terms of the functioning of the immune system. All marine species live in an environment rich in microorganisms and, therefore, actively use the immediate protection based on AMPs with different structures and biological properties, implemented in functions of the innate immune system. The role of AMPs is especially important for marine invertebrates, which represent the vast majority of the species diversity of marine organisms and do not have adaptive immunity [3]. It is worth noting that to date, at least 10 thousand species of marine polychaetes have been described [4, 5], each being a rich potential source of new peptide antibiotics, as evidenced by our previous studies [6–8]. Antimicrobial peptides from marine species are of significant practical interest, because these peptides retain their high antibacterial activity in the presence of salts over a wide range of concentrations: from physiological to those characteristic of sea water, at which the majority of cationic peptides, including the human cathelicidin LL-37, become much less active.
Isolation of new peptides and proteins from natural sources today remains a labor-intensive and time-consuming technology. Obviously, a more effective approach to the search for new AMPs is provided by the bioinformatics analysis of databases and in silico prediction of potential antimicrobial agents with their subsequent biological screening. At present more and more scientific groups are resorting to searching for new AMPs and bacteriocins in the databases of nucleotide sequences of genomes and transcriptomes [9, 10]. Previously, in our study of the structure of the arenicin precursor proteins from the sandworm Arenicola marina, we discovered the so-called BRICHOS domain (~100 aa), which is present in various animals, including humans [7, 11].
More and more evidence showing that BRICHOS domain acts as a molecular chaperone in the biosynthesis of relatively hydrophobic and amphiphilic molecules is being reported [12]. Recent results have suggested that the BRICHOS domain, like the cathelin-like domain in vertebrates [8, 13], may be involved in the biosynthesis of AMPs of different structural classes in polychaetes. The homology of amino acid sequences among the BRICHOS domain-containing proteins from different biological species is low (especially in comparison with the conserved cathelin-like domain), but however, the presence of two conserved regions in the structure makes it possible to search for sequences encoding BRICHOScontaining proteins in animal transcriptomes. Using this approach, we discovered a panel of new β-hairpin arenicin-like AMPs in the transcriptomes of a number of polychaetes, including A. marina [10], and also identified new structural families of AMPs in the polychaete Heteromastus [14].
The most common judgment about the mechanism of action of AMPs is based on the ability of most of them to disrupt the cytoplasmic membrane and kill target cells. However, one should take into account that some AMPs completely lack membranotropic properties. In recent years, more and more information has become available about the intracellular targets of AMPs (for example, the ribosome [15]), as well as about various molecular targets on the cell surface (for example, essential proteins of the outer membrane of Gram-negative bacteria [16] and structural components of the cell wall of Gram-positive bacteria [17]), which further reduces the risk of developing resistance to these compounds. Defensin-like AMPs stabilized by three or four disulfide bonds and resistant to proteolysis, which bind to structural elements of the bacterial cell wall, in particular lipid II, have a significant therapeutic potential as antibiotics for systemic use. Previously, this ability was found in human α- and β-defensins, defensins of the Cg-Def family of the Pacific oyster Crassostrea gigas, as well as plectasin from the fungus Pseudoplectania nigrella [17]. The goal of this work was the recombinant production of a new defensin-like antimicrobial peptide AmBRI-44a from the marine polychaete A. marina by heterologous expression in Escherichia coli cells and investigation of its biological activity and the mechanism of antibacterial action.
RESULTS AND DISCUSSION
Bioinformatic Search for BRICHOS-Related AMPs from the Marine Polychaete Arenicola marina
Stanovova et al. [18] in their recent in-depth study of gene expression in coelomocytes of the sandworm A. marina, related to the animal innate immune system, new BRICHOS-containing proteins with unknown function were discovered, in particular, identified a new cysteine-rich peptide, which was given the name AmIMP2 [18]. Unlike the known BRICHOS domain-containing precursors of AMPs, for example, preproarenicins [7], the AmIMP2 precursor protein contains in its structure a transmembrane (anchor) region, rather than a signal peptide (Fig. 1a). In this work, we continued the search for BRICHOS-related polychaete AMPs in our earlier studied A. marina transcriptome, which contains information on gene expression in all tissues of the animal (NCBI Accession no. SRX1015734). Together, the search for the presence of the BRICHOS domain in a database of translated sequences using HMMscan [19], as well as the BLAST search for homology of the AmIMP2 sequence, revealed two additional representatives of a new family of cysteine-rich BRICHOS-related peptides comprising 44 aa (including 8 cysteine residues) and named AmBRI44a and AmBRI-44b (Fig. 1b). These peptides are closely related to each other and differ only in two amino acid residues (L39M, S42R). Compared to AmIMP2, the new peptides have higher total positive charges (+3 and +4, respectively) at neutral pHs, an important characteristic indicative of the potential antimicrobial activity of cationic AMPs. Noteworthy is the unique structure of the new family of peptides: the search for antimicrobial peptides in the APD3 database [20] revealed a low degree of homology of the primary structure of the new peptides (< 40%) with known AMPs, and their closest homologs, in terms of the location of cysteine residues in the amino acid sequence, are plant AMPs of the hevein family, the main target of which is mold fungi [21].
Primary structure of new BRICHOS- related peptides from A. marina: (a) structural organization of precursor proteins of new cysteine-rich peptides: (TM) transmembrane (anchor) region, (KR) potential furin-like protease processing sites (specified as scissors), and (AMP) mature peptide; (b) alignment of amino acid sequences of the AmIMP2 peptide [18], new peptides AmBRI-44a and AmBRI44b from A. marina, and hevein from Hevea brasiliensis (UniProt: P02877). Cysteine residues are highlighted with a black background, arginine and lysine residues are highlighted with a gray background, and aspartic and glutamic acid residues are underlined. The square brackets indicate the arrangement of four disulfide bonds.
Recombinant Production of the New Peptide AmBRI-44a
For further structural and functional studies we chose the new peptide AmBRI-44a, because it is structurally closer to the previously described peptide AmIMP2. Considering the lack of data on the correct four disulfide bridge pairing of new peptides, we produced AmBRI44a as part of a thioredoxin A fusion protein in the E. coli BL21 (DE3) expression system. Thioredoxin A is widely used as a carrier protein, which ensures not only a reduction of the potential toxicity of the resulting peptide incorporated in the hybrid protein, but also correct folding, identical to natural, which was previously demonstrated by the production of recombinant analogs of a number of toxins and AMPs stabilized by disulfide bonds [22].
Thus, the AmBRI-44a peptide was produced as a part of the fusion protein including the following elements:
1) Eight histidine residues (His-tag) in the N-terminal part of the protein, which allowed the purification of the product by affinity chromatography;
2) Thioredoxin A sequence with the substitution of methionine for leucine (M37L);
3) Methionine residue;
4) Mature AmBRI-44a sequence.
The final purification of the target recombinant peptide AmBRI-44a after the specific cleavage of the hybrid protein with cyanogen bromide at the methionine residue was performed by reversed phase HPLC (RP-HPLC) on a semipreparative Reprosil-Pur C18-AQ column with linear gradient elution from 5 to 90% aqueous acetonitrile for 60 min (Fig. 2a).
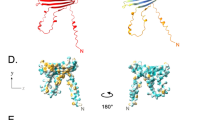
Recombinant production of the peptide AmBRI-44a and its structural characterization: (a) chromatogram of the purification of the AmBRI-44a peptide by RP-HPLC. The peptide peak was detected by changes in optical absorption at (gray line) 214 nm and (black line) 280 nm. The asterisk on the chromatogram indicates the fraction containing the target peptide; (b) MALDI mass spectrometric analysis of the RP-HPLC fraction corresponding to the target peptide; (c) circular dichroism (CD) spectra of the purified recombinant peptide AmBRI-44a in water and in dodecylphosphocholine (DPC) micelles; (d) analysis of the secondary structure of the AmBRI-44a peptide using CD spectroscopy data and CONTINLL [35]; (e) PyMOL visualization of the AlphaFold2 model of the spatial structure of the AmBRI-44a peptide (ColabFold [36]). Disulfide bonds are highlighted in black; (f) amino acid sequence of the AmBRI-44a peptide. Basic amino acid residues are highlighted in grey, and acidic residues are underlined. Square brackets indicate the arrangement of disulfide bonds (according to the predicted spatial structure of the peptide).
Analysis of the Structure of the AmBRI-44a Peptide
The main fraction of the eluate was analyzed by MALDI mass spectrometry (Fig. 2b). The experimental m/z value of the monoisotopic ion (5102.919) corresponded to the calculated molecular mass of the protonated ion of the target peptide ([M + H]+ 5103.17 Da) indicating formation of four disulfide bonds (–8 Da). The yield of the recombinant AmBRI-44a peptide was no less than 6 mg/L bacterial culture. Further, the structure of AmBRI-44a was analyzed by circular dichroism (CD) spectroscopy (Fig. 2c). The spectra were recorded in water, as well as in dodecylphosphocholine (DPC) micelles, a classic membrane-mimicking environment used in studies on cationic AMPs [23]. The CD spectrum of AmBRI-44a in water showed a well-defined minimum at 195 nm, an inflection at 200–205 nm, and a maximum at 225 nm. The significant difference from the spectrum patterns of hevein-like peptides in aqueous buffers (a maximum at 220 nm and a minimum at 200 nm [24]) suggests a radically different structure of AmBRI-44a, despite the fact that it has a similar arrangement of cysteine residues in the sequence. In the CD spectra of AmBRI-44a, measured in DPC micelles, two well-defined minima appear at 195 and 210 nm, implying a change in the conformation of the peptide in the micellar medium. Despite the obvious differences in the spectral patterns, an analysis of the CD data using the CONTINLL algorithm indicates a generally similar ratio of different types of secondary structure with the prevalence of β-strands and turns, as well as with the absence of α-helical elements (Fig. 2d), characteristic of heveins [21]. It is interesting to note that these results are in good agreement with the results of the de novo modeling of the spatial structure of AmBRI-44a using the AlphaFold2 algorithm (Fig. 2e). The resulting model is characterized by the presence of a central structural element, β-hairpin (20–36 aa), stabilized by a disulfide bond (Cys23–Cys33) and a typical β-turn (Asn28–Gly29). At the moment, the arrangement of disulfide bonds formation in AmBRI-44a was shown only by the modeling data (Fig. 2f). Thus, we suggest that AmBRI-44a is a predominantly β-structured peptide with a unique spatial organization that has never been described for AMPs before.
Antimicrobial Activity, Selectivity, and Cytotoxicity of the AmBRI-44a Peptide
At the next stage, we tested the antimicrobial activity of the AmBRI-44a peptide against a wide range of Gram-positive and Gram-negative bacteria and yeastlike fungi (Fig. 3a), as well as its potential toxic effect on mammalian cells (Fig. 3b).
The antimicrobial activity testing to determine the minimum inhibitory concentrations (MICs) was performed by two-fold serial dilutions in a liquid nutrient medium in accordance with microbiological standards. Screening the activity of AmBRI-44a against a wide panel of microorganisms (Table 1) revealed a specific effect of the peptide on bacteria of the genus Bacillus (strains B. subtilis B-886, B. licheniformis B-511, and B. mycoides B-814) and their related genus Mycobacterium (M. phlei Ac-1291). Activity against the Grampositive bacterial strain M. luteus B-1314 was detected only in a reduced-ionic-strength medium, providing evidence for a role of electrostatic repulsion in the interaction of the peptide with the target cell. It is worth noting that MICs of this peptide were not determined for a panel of Gram-negative bacterial strains, such as E. coli ML-35p, E. coli ATCC 25922, Pseudomonas aeruginosa PAO1, Klebsiella pneumonia ATCC 700603, Acinetobacter baumannii XDR CI 2675, Enterobacter cloacae XDR CI 4172, and Vibrio harveyi BB120, as well as Gram-positive bacterial strains, such as Staphylococcus aureus ATCC 6538P, S. aureus ATCC 29213, S. aureus MDR CI 119, Enterococcus faecalis ATCC 29212, and M. smegmatis mc(2)155 (MIC > 32 µM).
The AmBRI-44a peptide also showed activity against the yeast-like fungi Candida albicans, but the relatively high MIC values (16 µM) more likely point to a nonspecific effect on the membranes of these eukaryotic cells. Bruno et al. [25] observed a similar selective effect on a number of Gram-positive bacteria in combination with the nonspecific moderate activity against different eukaryotic cells, including yeast-like and mold fungi, with a number of defensin-like peptides of animal origin. The lack of selective antimicrobial activity is one of the significant disadvantages of many cationic AMPs acting on membranes of any composition. The toxic properties of AmBRI-44a were assessed against freshly isolated healthy red blood cells (hRBCs) and a standard transformed human embryonic kidney cell line (HEK293T). The peptide showed moderate hemolytic activity only at concentrations higher than 64 μM, which is also accompanied by erythrocyte agglutination. However, the AmBRI-44a peptide did not damage HEK293T cells even at a concentration of 64 μM.
We suggested that the peptide is inhibited in the presence of fetal bovine serum (FBS) in the culture medium, as previously shown for a number of AMPs [10]. Additional analysis of the activity of AmBRI-44a against the sensitive B. licheniformis B-511 test strain in the Mueller–Hinton medium (MHB) containing 5% FBS revealed no loss of activity (MIC ~1 μM). Thus, we can conclude that AmBRI-44a has a moderate cytotoxic effect on mammalian cells, which is observed only at concentrations two orders of magnitude higher than the MIC values for the most sensitive bacteria.
Mechanism of Action of the AmBRI-44a Peptide. Induction of Bacterial Resistance to the AmBRI-44a Peptide
Such a selective action of AmBRI-44a, not associated with membrane disruption, suggests the presence of a specific target on the surface of some species of Grampositive bacteria, primarily those belonging to the genus Bacillus. In order to identify the potential target for AmBRI-44a, we performed a 30-day selection of a resistant strain of B. licheniformis B-511 bacteria by successive daily subculturing (passaging) of the cell culture, using 96-well plates in the Mueller–Hinton medium containing the test antimicrobial drugs in concentrations obtained by two-fold serial dilutions.
This method allowed monitoring changes in the MIC values of the test drugs with every subculturing (Fig. 4a). As a result, we obtained a B. licheniformis strain (RES) resistant to the AmBRI-44a peptide and showed that the MIC of the peptide increased 16-fold after 27 passagings. After 3-day cultivation in the medium without selection pressure (in the absence of AMP), the resulting strain did not restore sensitivity to this peptide (the final increase of the MIC was 8 times), which indicates the development of a stable bacterial resistance and the presence of corresponding mutations in the genomic DNA. It is important to note that for AMPs, whose action is predominantly associated with bacterial cell membrane disruption regardless of the mode of action (detergent-like, toroidal pore, or another mechanism), as a rule, it is impossible to select strains with stable resistance, because such an adaptation is too costly for cellular physiology. With the strain resistant to the AmBRI-44a peptide, no significant differences were observed in the growth rate of the bacterial culture, but significant differences were found in the shape of the colonies: in contrast to the natural phenotype, which is characterized by an irregular lichen-like shape with abundant hair-like outgrowths, the resistant strain had exclusively round-shaped colonies without any outgrowths, which is not typical of B. licheniformis.
Plausible mechanism of the antibacterial action of the AmBRI-44a peptide: (a) induction of resistance of B. licheniformis B-511 bacteria to the AmBRI-44a peptide (initial MIC value 0.25 µM). After 27 days of successive passages in the presence of the AmBRI-44a peptide, a bacterial culture capable of growing at the highest concentration of AMPs was subcultured on the MHB agar medium for 3 days, after which the final MIC value was determined; (b) structural organization of the sensor histidine kinase WalK of B. licheniformis bacteria, which consists of five domains: (PAS*, PER-ARNT-SIM sensor) extracellular sensor domain and (HAMP, the abbreviation reflects the presence of the domain in the following proteins: Histidine kinases, Adenylyl cyclases, Methyl-accepting chemotaxis proteins, and Phosphatases) cytoplasmic domain responsible for phosphorylation during signal transduction; (PAS) cytoplasmic sensory domain; (HisKA) dimerizing domain responsible for histidine autophosphorylation; and (HATPase) ATPase domain of histidine kinase; and (c) alignment of amino acid sequences of the HAMP domain of histidine kinase WalK for various bacteria. The previously discovered residues, evidence for whose polymorphism and the corresponding mutant phenotype of S. aureus resistance to vancomycin was obtained in [32, 37]) are highlighted in black. The WalK[R217S] mutation found in B. licheniformis in this study is highlighted in gray.
To identify genetic changes in the resistant B. licheniformis strain, we performed genome sequencing. Analysis of the resulting data allowed us to detect a polymorphism, specifically two single-nucleotide mutations, compared to the wild-type strain. The first mutation leads to a V375A amino acid substitution in the poorly studied TgpA protein, which, according to the SMART analysis [26], is located in the cytoplasmic membrane and contains a domain with transglutaminase activity. The BLAST analysis in genomic databases showed that this polymorphism is characteristic of many natural strains of B. licheniformis.
Thus, the effect of this mutation on resistance to AmBRI-44a, while not excluded, is unlikely to play a key role. The second our discovered mutation in the resistant strain of B. licheniformis leads to an R217S substitution in such a vitally important protein for bacilli as the WalK protein [27], a histidine kinase of the WalK/ WalR two-component system (TCS) (Fig. 4b), which regulates the biosynthesis of the cell wall of many Grampositive bacteria, in particular staphylococci and bacilli [28]. A number of studies showed that the WalK/WalR system regulates cell wall metabolism during cell growth, perceiving as a signal the appearance of peptidoglycan hydrolysis products, in particular, the D-Ala-D-Ala dipeptide, one of the structural elements of lipid II [29].
Thus, two possible hypotheses for the mechanism of action of AmBRI-44a can be considered:
1) direct interaction of the peptide with the WalK protein;
2) interaction with other targets, the biosynthesis of which is regulated by the WalK protein, primarily with cell wall components.
A number of natural antibiotics that directly act on the histidine kinase WalK are described in the literature. Thus, it was shown that valdiomycin and signermycin B, produced by bacteria of the genus Streptomyces, are able to bind to the HisKA domain (Fig. 4b) [30, 31]. Unlike the above-mentioned relatively small antibiotics, the AmBRI-44a peptide, having the molecular weight of >5 kDa, cannot penetrate the membrane without disrupting its structure. Furthermore, given the submicromolar MIC value of the peptide against B. licheniformis, a mechanism involving direct interaction of AmBRI-44a with the cytoplasmic domain of HAMP seems unlikely.
Several mutations previously identified in the HAMP domain of the WalK protein in S. aureus and associated with reduced sensitivity to vancomycin in bacteria are worthy of noting (Fig. 4c). Mutations in this domain also lead to thickening of the cell wall and decreased autolytic activity [32, 33], and, therewith, the overexpression of the walK and walR genes, too, lead to similar phenotypic changes. Since the selection of resistance of Grampositive bacteria to various inhibitors of cell wall biosynthesis often gives rise to a mutation in the walK gene, at the next stage we decided to analyze the effects of cross-resistance of the resulting B. licheniformis strain to various antibiotics, mainly targeting Gram-positive bacteria, as well as to AMPs (α-helical linear human cathelicidin LL-37 and β-hairpin tachyplesin-1 from the horseshoe crab Tachypleus tridentatus), whose action is associated with bacterial membrane disruption (Table 1).
As expected, cross-resistance (4-fold increase in MIC, highlighted in bold in Table 2) was detected only to vancomycin and nisin, which act through binding to lipid II. It is worth noting that the mechanisms of action of vancomycin and nisin are slightly different: vancomycin inhibits cell wall growth by binding to the C-terminal D-Ala-D-Ala motif of lipid II, while nisin binds to the pyrophosphate moiety of lipid II with the subsequent formation of a pore in the membrane [17]. Thus, the discovered mutation in the HAMP domain associated with decreasing sensitivity to AMPs, vancomycin and nisin, is the first case described for bacteria of the genus Bacillus, and the resulting evidence suggests that the putative mechanism of action of the AmBRI-44a peptide consists in inhibiting cell wall biosynthesis.
EXPERIMENTAL
Recombinant production of peptide AmBRI-44a. The first stage involved the construction of primers for the synthesis and amplification of the sequence encoding the target AMP, considering the E. coli codon usage bias. The AMP-coding sequence is preceded by the methionine ATG codon and followed by the stop codon TAA. The sequences of all primers were optimized using OligoAnalyzer (IDT; https://eu.idtdna.com/pages) to decrease the probability of the formation of secondary structures (hairpins) and dimers during synthesis. The sequence encoding the target AMP was obtained by slowly annealing of the 3′ ends (20 nucleotides each) of two primers corresponding to the N- and C-terminal regions of the AmBRI-44a peptide, followed by the extension to the double-stranded structure. The resulting DNA fragment was treated with the restriction endonucleases BglII and EcoRI (Thermo Scientific, USA) to form sticky ends.
After the purification of the linearized expression vector (plasmids based on the pET vector [23]), a ligase reaction was performed with the assembled DNA fragment. The resulting reaction mixture was used to transform E. coli DH10B cells by the heat shock method. Clones containing the required insert were selected by PCR using a thermostable Taq DNA polymerase (Evrogen, Russia). Amplification of the insert was carried out on a Tertsik programmable amplifier (DNA-Technology, Russia) using the T7 Reverse primer complementary to the transcription termination site, as well as the forward primer (5′-GGTCCGTGCAAACTGATCGCCCCGA-3′), complementary to the fragment of the gene encoding thioredoxin A. The temperature regime of the reaction was as follows: 95°С for 10 min (1 cycle), then 94°С for 30 s, 50°С for 40 s, and 72°С for 30 s (25 cycles). The correctness of the assembly of the intermediate and target constructs was confirmed by Sanger sequencing of the purified plasmid DNA.
The recombinant peptide was expressed in E. coli BL21 (DE3) cells as a fusion protein containing a His tag, thioredoxin A with M37L substitution, a methionine residue, and the AmBRI-44a sequence. Cells transformed with the corresponding plasmid were grown in the LB lysogenic medium supplemented with 20 mM glucose, 1 mM MgSO4, and 100 μg/mL ampicillin at 37°C to an optical density OD600 of 0.8–1.0. Gene expression was induced by adding 0.2 μM isopropyl-β-D-1-thiogalactopyranoside (IPTG), and the culture was incubated for 4–5 h at 30°C with vigorous agitation. The cells were harvested by centrifugation and sonicated in a buffer containing 100 μM NaH2PO4, 6 M guanidine–HCl, and 20 μM imidazole (pH 7.8). The clarified lysate was purified by metal chelate chromatography on Ni-NTA Sepharose (GE Healthcare, USA). The hybrid protein was eluted with a buffer containing 100 μM NaH2PO4, 6 M guanidine–HCl, and 0.5 M imidazole (pH 7.8). To isolate the target peptide, the collected fraction was titrated with conc. HCl (to pH 1.0–2.0), after which a 100-fold molar excess of BrCN was added. After 18 h of incubation in the dark at 25°C, the reaction was terminated by adding a 3-fold volume of water and evaporating the sample in a vacuum centrifuge at 37°C. The obtained solution was loaded on a reverse phase semipreparative ReproSil-Pur 120 C18-AQ column (10 × 250 mm, particle size 5 μM, Dr. Maisch, Germany) at a flow rate of 2 mL/min in a linear gradient of aqueous acetonitrile containing 0.1% TFA. The peptide elution was monitored at 214 and 280 nm, and the fraction was collected and analyzed on a Reflex III mass spectrometer (Bruker Daltonics, Germany). The fraction corresponding to the molecular weight of the target peptide AmBRI-44a was dried on a SpeedVac vacuum concentrator (Savant, USA) and dissolved in water. The peptide concentration was assessed on a spectrophotometer (Implen, Germany) by absorbance at 280 nm.
Analysis of the secondary structure of the AmBRI44a peptide by circular dichroism spectroscopy. The CD spectra of AmBRI-44a were measured on a J-810 spectropolarimeter (Jasco, Japan) at the Center for Collective Use of the Institute of Bioorganic Chemistry, Russian Academy of Sciences. The measurements were performed in triple distilled water or a 30 μM dodecylphosphocholine (DPC) within a range from 190 to 250 nm in quartz cells with an optical path length of 0.01 cm. The averaged data of four measurements for each sample were analyzed using tCONTINLL (CDPro package; https://www.lamar.colostate.edu/~sreeram/CDPro).
Analysis of the antibacterial activity of the AmBRI44a peptide. The antibacterial activity of the AmBRI44a peptide was assessed by the two-fold serial dilution method using sterile 96-well flat-bottomed polystyrene microplates (Eppendorf #0030730011) in the Mueller–Hinton medium (MHB; Sigma, USA) in the presence or absence of 0.9% NaCl. Bacterial cultures (Table 1) were grown in the LB medium until an optical density OD600 of 1.0 at 37°C, after which they were diluted by 2 × MHB ± 1.8% NaCl to a final cell concentration of 106 CFU/mL. Aliquots of the test cultures (50 μL) were added to 50 μL of an aqueous solution of AmBRI-44a peptide preliminarily dissolved in 0.1% sterile bovine serum albumin (BSA) to reduce its nonspecific binding to the plate surface. The plates were incubated for 24 h at 37°C and 950 rpm on a microplate shaker. The MIC values were determined using a spectrophotometer (Implen, Germany) as the minimum concentrations of the AmBRI-44a peptide, at which no culture growth took place, and were calculated as the median of the values obtained in three independent experiments.
Analysis of the antifungal activity of the AmBRI44a peptide. The antifungal activity of the AmBRI-44a peptide was assessed by the two-fold serial dilution method using sterile 96-well microplates. Cultures of yeast-like fungi C. albicans (Table 1) were grown on sterile Petri dishes with modified YPD agar (yeast extract 5 g/L, peptone 10 g/L, and glucose 10 g/L) for 48 h at 37°C and then cultured in modified YPD broth at 37°C until an optical density of 1.0 at a wavelength of 570 nm. The yeast cell suspension was diluted with standard RPMI medium to a final concentration of 5 × 104 CFU/mL and mixed with an equal volume of an aqueous solution of the AmBRI-44a peptide. The plate was incubated at 30°C for 24 h. Yeast growth was assessed visually using a CX31 microscope (Olympus, Germany), as well as by measuring the optical density of the culture in the wells at 570 nm. The MIC values were determined as the lowest concentration of the AmBRI-44a peptide able to inhibit culture growth and calculated as the median of the values obtained from two independent experiments.
Analysis of the hemolytic activity and cytotoxicity of the AmBRI-44a peptide. The ability of AMP to disrupt eukaryotic membranes was assessed on healthy donor red blood cells (hRBC) and transformed human embryonic kidney cells (HEK293T), obtained from the Institute of Cytology of the Russian Academy of Sciences. Citrate buffer was added to the collected healthy donor blood samples to prevent clotting. Subsequently, the whole blood was centrifuged in a solution of Ficoll 400 and urografin (density 1.077 g/mL) for 15 min at 500 g. The red blood cell fraction was collected from the bottom and washed three times with twenty volumes of chilled phosphate-buffered saline (PBS, pH 7.4). For the assay in a 96-well plate, a series of two-fold dilutions of the test peptide from 64 to 2 μM (in terms of the final concentration in the well) was prepared in 50 mL of a 0.1% BSA solution. After this, 50 μL of an 8% suspension of red blood cells in PBS was added to the peptide solution. The red blood cell suspension was incubated with AmBRI-44a for 1.5 h at 37°C and 950 rpm. After incubation, the plate was centrifuged for 15 min at 1000 g to precipitate intact red blood cells. Aliquots of the supernatant were transferred to a new plate, and the released hemoglobin was measured spectrophotometrically at 405 nm. A suspension of red blood cells in PBS and 0.1% Triton X-100 were used as negative and positive controls, respectively.
The hemolytic activity of the AmBRI-44a peptide was calculated by the formula (%):
The cytotoxic effect of AmBRI-44a was assessed using MTT assay, which is based on the ability of living cell dehydrogenases to reduce the yellow reagent MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2Htetrazolium bromide) to the water-insoluble purple crystalline formazan. Cells were seeded onto a 96-well plate (104 cells per well) in a modified DMEM/F12 medium supplemented with 10% fetal bovine serum (FBS), and the plate was placed in a CO2 incubator (5% CO2, 37°C). After incubation for 24 h, the culture liquid was replaced with a solution of the test peptide AmBRI-44a in a fresh medium. After 16-h incubation, 20 μL of MTT solution (5 mg/mL) was added to each well, and incubation was continued for an additional 4 h. The medium was carefully removed, and 100 μL of a 1 : 1 mixture of DMSO and ethanol was added to each well to dissolve formazan crystals. The optical density of solutions was measured at 570 nm using an AF2200 microplate reader (Eppendorf, Germany). The optical density of the cell solution without the addition of the peptide was taken as 100% viable cells.
Induction of bacterial resistance to the AmBRI44a peptide. The development of resistance to AmBRI44a was studied by serial subculturing of the bacterial culture of B. licheniformis B-511 using 96-well plates in Mueller–Hinton broth (MHB) containing the test peptide at concentrations obtained by two-fold serial dilutions. To prepare the starting inoculum, a pure daily culture of the test strain grown on an agar nutrient medium was used. Several individual colonies were picked with a bacterial loop and inoculated into a 15 mL tube containing 6 mL of MHB medium. The tube was incubated at 37°C under shaking with a speed of 220 rpm for 20 h until the culture reached the stationary growth phase. The culture was diluted 500 times with fresh two-fold MHB medium, after which 50 μL aliquots of the resulting bacterial suspension were added to 50 μL of a solution of AmBRI-44a serially diluted in 0.1% BSA in a 96-well microplate (Eppendorf #0030730011). The plate was incubated at 37°C with vigorous agitation at 950 rpm for 22 ± 2 h. The MIC values were determined as the lowest concentration of the test peptide, at which no visible bacterial growth took place. Then, every day, 2 μL of the bacterial suspension was taken from a well containing the AmBRI-44a peptide at a subinhibitory concentration as close as possible to the MIC and diluted with 1 mL of fresh two-fold MHB medium. The resulting suspension was added in 50 μL aliquots to another plate to 50 μL of a solution of the test peptide, serially diluted to concentrations from 0.25× to 16× current MIC values. The passages were repeated for 27 days, after which three successive subculturings of the resulting strain on a solid Mueller–Hinton medium without AmBRI-44a peptide were performed.
Analysis of genetic polymorphisms of the bacterial strain B. licheniformis resistant to the AmBRI-44a peptide. Genomic DNA sequencing of B. licheniformis B-511 strains resistant to the AmBRI-44a peptide, as well as the wild-type control strain, was carried out by Biospark LLC (Troitsk, Russia) on the Illumina MiSeq platform (USA). The quality of the resulting paired-end reads (100 × 2) was assessed using FastQC (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Low-quality reads, as well as reads containing adapter sequences, were filtered using Trimmomatic (http://www.usadellab.org/cms/?page=trimmomatic), after which quality control was repeated. The genome of the control strain was assembled using SPAdes (URL: www.cab.spbu.ru/software/spades/) using high-quality paired and unpaired reads. The quality of assembly was assessed using QUAST (https://bioinf.spbau.ru/quast). Structural annotation of the genome of the control strain was performed using Prokka (https://github.com/kbaseapps/ProkkaAnnotation). Alignments of the DNA reads from the resistant strain onto the genome assembly of the control strain, as well as to work with these alignments, the BWA (https://bio-bwa.sourceforge.net/) and SAMtools (https://samtools.sourceforge.net/) toolkits were used. Strain polymorphisms were analyzed using VarScan (https://varscan.sourceforge.net/); polymorphisms with the coverage share higher than 0.9 were considered true.
Analysis of the cross-resistance of the selected B. licheniformis strain to conventional antibiotics and various AMPs. The effects of cross-resistance of the selected B. licheniformis B-511 strain to various antimicrobial agents were determined by two-fold serial dilutions in sterile 96-well flat-bottomed polystyrene microplates (Eppendorf #0030730011) in Mueller–Hinton broth (MHB; Sigma, USA) in the absence of 0.9% NaCl as above. Cathelicidin LL-37 was obtained by solid-phase synthesis and was kindly provided by M.N. Zhmak (purity > 98%); tachyplesin-1 was obtained by heterologous expression in E. coli bacterial system; nisin (Sigma, USA); vancomycin (AppliChem, Germany); rifampicin (Sigma, USA); tetracycline (Sigma, USA); gentamicin (AppliChem, Germany).
Statistical data analysis. All data were obtained in at least two independent experiments with at least two replicates and are presented with the standard deviation of mean values. Statistical analysis was performed using Graphpad Prism 6.0 (GraphPad Software) and Student’s t-test, and values with p < 0.05 were considered statistically significant.
CONCLUSIONS
Here, we describe identification and biological properties evaluation of a novel BRICHOS-related antimicrobial peptide AmBRI-44a from the lugworm Arenicola marina. It represents a previously undiscovered in polychaetes a structural family of defensin-like peptides stabilized by four disulfide bonds. The specific activity of AmBRI-44a peptide against bacteria of the genera Bacillus and Mycobacterium was revealed and studied, which is of significant interest from a fundamental viewpoint. Bacilli are known to produce many antimicrobial compounds and are part of the symbiotic microbiota of a number of marine invertebrates [29].
One possible function of AmBRI-44a and related peptides may be to control the species composition of the microbiome, rather than to fight pathogens. The low level of expression of the homologous peptide AmIMP2 [18] is presumably associated with the peculiar features of its biosynthesis. For example, an inducible biosynthesis of AmIMP2 and AmBRI-44a in response to the presence of target cells, which is characteristic of narrowacting peptides of invertebrates, cannot be excluded [1]. In terms of plausible mechanism of action, the selectivity of the peptide against Bacillus and Mycobacterium may be associated with the special structure of lipid II in these bacteria as a potential target of AmBRI-44a: unlike what is observed in the majority of Gram-positive bacteria, the third amino acid residue in their pentapeptide is represented by meso-diaminopimelic acid (meso-DAPC, DAP) instead of L-lysine [34].
The resulting data open up further directions for research into the discovered new family of defensin-like BRICHOS-related polychaete AMPs; in particular, to verify the hypothesis that their mechanism of action is associated with the inhibition of cell wall biosynthesis is of particular interest. Evidence for this assumption can be obtained with the use of both biochemical approaches and molecular modeling and structural biology. Equally important is to further study of the physiological role of the AmBRI-44a peptide and its localization in the body of the sandworm Arenicola marina.
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
Lazzaro, B.P., Zasloff, M., and Rolff, J., Science, 2020, vol. 368, Article ID: eaau5480. https://doi.org/10.1126/science.aau5480
Guryanova, S.V. and Ovchinnikova, T.V., Mar. Drugs, 2022, vol. 20, Article ID: 549. https://doi.org/10.3390/md20090549
Guryanova, S.V., Balandin, S.V., Belogurova-Ovchinnikova, O.Yu., and Ovchinnikova, T.V., Mar. Drugs, 2023, vol. 21, Article ID:503. https://doi.org/10.3390/md21100503
Mora, C., Tittensor, D.P., Adl, S., Simpson, A.G., and Worm, B., PLoS Biol., 2011, vol. 9, Article ID: e1001127. https://doi.org/10.1371/journal.pbio.1001127
World Register of Marine Species. https://www.marinespecies.org
Panteleev, P.V., Tsarev, A.V., Safronova, V.N., Reznikova, O.V., Bolosov, I.A., Sychev, S.V., Shenkarev, Z.O., and Ovchinnikova, T.V., Mar. Drugs, 2020, vol. 18, Article ID: 620. https://doi.org/10.3390/md18120620
Ovchinnikova, T.V., Aleshina, G.M., Balandin, S.V., Krasnosdembskaya, A.D., Markelov, M.L., Frolova, E.I., Leonova, Y.F., Tagaev, A.A., Krasnodembsky, E.G., and Kokryakov, V.N., FEBS Lett., 2004, vol. 577, pp. 209–214. https://doi.org/10.1016/j.febslet.2004.10.012
Panteleev, P.V., Tsarev, A.V., Bolosov, I.A., Paramonov, A.S., Marggraf, M.B., Sychev, S.V., Shenkarev, Z.O., and Ovchinnikova, T.V., Mar. Drugs, 2018, vol. 16, Article ID: 401. https://doi.org/10.3390/md16110401
Metelev, M., Osterman, I.A., Ghilarov, D., Khabibullina, N.F., Yakimov, A., Shabalin,, K., Utkina, I., Travin, D.Y., Komarova, E.S., Serebryakova, M., Artamonova, T., Khodorkovskii, M., Konevega, A.L., Sergiev, P.V., Severinov, K., and Polikanov, Y.S., Nat. Chem. Biol., 2017, vol. 13, pp. 1129–1136. https://doi.org/10.1038/nchembio.2462
Safronova, V.N., Bolosov, I.A., Kruglikov, R.N., Korobova, O.V., Pereskokova, E.S., Borzilov, A.I., Panteleev, P.V., and Ovchinnikova, T.V., Mar. Drugs, 2022, vol. 20, Article ID: 517. https://doi.org/10.3390/md20080517
Presto, J. and Johansson, J., The BRICHOS Domain. Its Proproteins and Functions, Heidelberg, Springer, 2015, pp. 1–28. ISBN 978-3-319-16563-9
Leppert, A., Poska, H., Landreh, M., Abelein, A., Chen, G., and Johansson, J.A., Protein Sci., 2023, vol. 32, Article ID: e4645. https://doi.org/10.1002/pro.4645
Bruno, R., Boidin-Wichlacz, C., Melnyk, O., Zeppilli, D., Landon, C., Thomas, F., Cambon, M.-A., Lafond, M., Mabrouk, K., Massol, F., Hourdez, S, Maresca, M., Jollivet, D., and Tasiemski, A., Sci. Total Environ., 2023, vol. 879, Article ID: 162875. https://doi.org/10.1016/j.scitotenv.2023.162875
Panteleev, P.V., Safronova, V.N., Duan, S., Komlev, A.S., Bolosov, I.A., Kruglikov, R.N., Kombarova, T.I., Korobova, O.V., Pereskokova, E.S., Borzilov, A.I., Dyachenko, I.A., Shamova, O.V., Huang, Y., Shi, Q., and Ovchinnikova, T.V., Mar. Drugs, 2023, vol. 21, Article ID: 639. https://doi.org/10.3390/md21120639
Graf, M., Mardirossia, M., Nguyen, F., Seefeldt, A.C., Guichard, G., Scocchi, M., Innis, C.A., and Wilson, D.N., Nat. Prod. Rep., 2017, vol. 34, pp. 702–711. https://doi.org/10.1039/c7np00020k
Vetterli, S.U., Zerbe, K., Müller, M., Urfer, M., Mondal, M., Wang, S.-Y., Moehle, K., Zerbe, O., Vitale, A., Pessi, G., Eberl, L., Wollscheid, B., and Robinson, J.A., Sci. Adv., 2018, vol. 4, eaau2634. https://doi.org/10.1126/sciadv.aau2634
Grein, F., Schneide, T., and Sahl, H.-G., J. Mol. Biol., 2019, vol. 431, pp. 3520–3530. https://doi.org/10.1016/j.jmb.2019.05.014
Stanovova, M.V., Gazizova, G.R., and Gorbushin, A.M., J. Exp. Zoolog. B: Mol. Dev. Evol., 2023, vol. 340, pp. 34–55. https://doi.org/10.1002/jez.b.23135
Potter, S.C., Luciani, A., Eddy, S.R., Park, Y., Lopez, R., and Finn, R.D., Nucleic Acids Res., 2018, vol. 46, pp. W200–W204. https://doi.org/10.1093/nar/gky448
Wang, G., Li, X., and Wang, Z., Nucleic Acids Res., 2016, vol. 44, pp. D1087–D1093. https://doi.org/10.1093/nar/gkv1278
Slezina, M.P. and Odintsova, T.I., Curr. Issues Mol. Biol., 2023, vol. 45, pp. 3674–3704. https://doi.org/10.3390/cimb45040239
Shenkarev, Z.O., Panteleev, P.V., Balandin, S.V., Gizatullina, A.K., Altukhov, D.A., Finkina, E.I., Kokryakov, V.N., Arseniev, A.S., and Ovchinnikova, T.V., Biochem. Biophys. Res. Commun., 2012, vol. 429, pp. 63–69. https://doi.org/10.1016/j.bbrc.2012.10.092
Panteleev, P.V., Bolosov, I.A., Balandin, S.V., and Ovchinnikova, T.V., J. Pept. Sci., 2015, vol. 21, pp. 105–113. https://doi.org/10.1002/psc.2732
Rodríguez-Romero, A., Arreguín, B., and Hernández-Arana, A., Biochim. Biophys. Acta, 1989, vol. 998, pp. 21–24. https://doi.org/10.1016/0167-4838(89)90113-1
Bruno, R., Maresca, M., Canaan, S., Cavalier, J-F., Mabrouk, K., Boidin-Wichlacz, C., Olleik, H., Zeppilli, D., Brodin, P., Massol, F., Jollivet, D., Jung, S., and Tasiemski, A., Mar. Drugs, 2019, vol. 17, p. 512. https://doi.org/10.3390/md17090512
Letunic, I., Khedkar, S., and Bork, P., Nucleic Acids Res., 2021, vol. 49, pp. D458–D460. https://doi.org/10.1093/nar/gkaa937
Koo, B.-M., Kritikos, G., Farelli, J.D., Todor, H., Tong, K., Kimsey, H., Wapinski, I., Galardini, M., Cabal, A., Peters, J.M., Hachmann, A.-B., Rudner, D.Z., Allen, K.N., Typas, A., and Gross, C.A., Cell Syst., 2017, vol. 4, pp. 291–305.e7. https://doi.org/10.1016/j.cels.2016.12.013
Dubrac, S., Bisicchia, P., Devine, K.M., and Msadek, T.A., Mol. Microbiol., 2008, vol. 70, pp. 1307–1322. https://doi.org/10.1111/j.1365-2958.2008.06483.x
Blockley, A., Elliott, D., Roberts, A., and Sweet, M., Diversity, 2017, vol. 9, Article ID: 49. https://doi.org/10.3390/d9040049
Kato, A., Ueda, S., Oshima, T., Inukai, Y., Okajima, T., Igarashi, M., Eguchi, Y., and Utsumi, R., J. Gen. Appl. Microbiol., 2017, vol. 63, pp. 212–221. https://doi.org/10.2323/jgam.2016.10.007
Watanabe, T., Igarashi, M., Okajima, T., Ishii, E., Kino, H., Hatano, M., Sawa, R., Umekita, M., Kimura, T., Okamoto, S., Eguchi, Y., Akamatsu, Y., and Utsumi, R., Antimicrob. Agents Chemother., 2012, vol. 56, pp. 3657–3663. https://doi.org/10.1128/AAC.06467-11
Zhu, J., Liu, B., Shu, X., and Sun, B., Int. J. Med. Microbiol., 2021, vol. 311, Article ID: 151473. https://doi.org/10.1016/j.ijmm.2021.151473
Jaumaux, F., Petit, K., Martin, A., Rodriguez-Villalobos, H., Vermeersch, M., Perez-Morga, D., and Gabant, P., Antibiotics, 2023, vol. 12, p. 947. https://doi.org/10.3390/antibiotics12060947
Vollmer, W., Blanot, D., and de Pedro, M.A., FEMS Microbiol. Rev., 2008, vol. 32, pp. 149–167. https://doi.org/10.1111/j.1574-6976.2007.00094.x
Provencher, S.W. and Glöckner, J., Biochemistry, 1981, vol. 20, pp. 33–37. https://doi.org/10.1021/bi00504a006
Mirdita, M., Schütze, K., Moriwaki, Y., Heo, L., Ovchinnikov, S., and Steinegger, M., Nat. Methods, 2022, vol. 19, pp. 679–682. https://doi.org/10.1038/s41592-022-01488-1
Baseri, N., Najar-Peerayeh, S., and Bakhshi, B., BMC Microbiol., 2021, vol. 21, p. 240. https://doi.org/10.1186/s12866-021-02298-9
Funding
The work was financially supported by the Russia Science Foundation (project no. 22-14-00380; https://rscf.ru/project/22-14-00380/).
Author information
Authors and Affiliations
Contributions
The authors TVO and PVP—formulated the concept of the research; the authors VNS, IAB, PVP, RNK, and EIF—accomplished the experimental part of the work; the authors VNS, PVP, and TVO analyzed the results and prepared the manuscript for publication and edited it.
The final version of the manuscript was approved by all authors.
Corresponding author
Ethics declarations
This article contains no description of any research involving humans and animals as research subjects.
The blood samples used to determine the hemolytic activity of the peptide were obtained from a healthy donor.
Informed consent was obtained in writing. No conflict of interest was declared by the author.
Additional information
Publisher's Note. Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations: AMP, antimicrobial peptides; RP-HPLC, reversed-phase HPLC; CD, circular dichroism; DPC, dodecylphosphocholine; MIC, minimum inhibitory concentration; FBS, fetal bovine serum; MHB, Mueller–Hinton medium.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Safronova, V.N., Panteleev, P.V., Kruglikov, R.N. et al. Novel BRICHOS-related Defensin-like Antimicrobial Peptide from the Marine Polychaeta Arenicola marina. Russ J Bioorg Chem 50, 629–643 (2024). https://doi.org/10.1134/S1068162024030087
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162024030087