Abstract
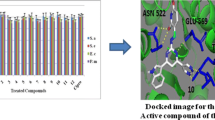
Objective: This article is focused on synthesis and characterisation of 1,1-(((5-(3,4,5triethoxybenzyl)pyrimidine-2,4-diyl))bis(azanediyl))bis(substituted phenyl methylene))bis(naphthalen-2-ol) moieties and evaluated for antibacterial and antioxidant activities as well as molecular docking and molecular dynamic simulation investigations. Methods: In this article we conducted one-pot three component reactions through Betti type reaction. Agar diffusion method was used to evaluate anti-bacterial properties. The DPPH method was used to determine antioxidant activity results. Results: A series of 1,1-(((5-(3,4,5triethoxybenzyl)-pyrimidine-2,4-diyl))bis(azanediyl))bis(substituted phenyl methylene))bis(naphthalen-2-ol) were synthesized via Betti type reaction and evaluated for their antibacterial and antioxidant activities. Further, all the synthesized compounds were characterized by FT-IR, 1H, and 13C NMR, and mass spectral analysis. Discussion: Molecular docking was carried out to study possible potential binding interaction of synthesized Betti base derivatives with 1NC6 PDB as receptor. The molecular dynamic (MD) was performed to investigate stability of binding interaction of drug-target (protein–ligand) complex. All the synthesized compounds were investigated for their antibacterial and antioxidant activity. Conclusions: Betti base derivatives were synthesized and studied on the basis of computer aided drug design including docking and molecular dynamic (MD) simulations and pharmacologically assessed for antioxidant and antibacterial activity. Molecular docking investigation revealed that compounds showed better binding energies in the range –4.1 to –7.3 kcal/mol suggests that the binding occurs efficiently in the receptor–ligand complex. These Betti base derivatives were synthesized via Betti base protocol at 70oC through one-pot three component reaction with acceptable yields, as cost efficient, high yield, easy workup and environmental friendliness. Afterwards, obtained molecules have characterized by FT-IR, NMR, and Mass spectra which confirms the chemical structure of the synthesized compounds.









Similar content being viewed by others
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
Karmakar, B. and Banerji, J., Tetrahedron Lett., 2011, vol. 52, pp. 4957–4960. https://doi.org/10.1016/j.tetlet.2011.07.075
Ugi, I., Werner, B., and Domling, A., Molecules, 2003, vol. 8, pp. 53–66. https://doi.org/10.3390/80100053
Betti, M., Org. Synth. Coll., 1941, vol. 1, pp. 381–384.
Martinek, A., Tetrahedron, 2003, vol. 59, pp. 2877–2884. https://doi.org/10.1016/S0040-4020(03)
Periasamy, M., Reddy, M.N., and Anwar, S., Tetrahedron Assymmet., 2004, vol. 15, pp. 1809–1812. https://doi.org/10.1016/j.tetasy.2004.04.030
Mohanram, I. and Meshram, J., ISRN Org. Chem., 2014, vol. 2014, Article ID: 639392. https://doi.org/10.1155/2014/639392
Monsouri, S.G., Z-Boeini, H., Zomorodian, K., Khalvati, B., Pargali, R.H., Dehsharhi, A., Rudbari, H.A., Sahihi, M., and Chavoshpour, Z., Arab. J. Chem., 2020, vol. 13, pp. 1271–1282. https://doi.org/10.1016/j.arabjc.2017.10.009
Mallamaci, R., Annunziata, M., Capozzi, M., and Cosimocardellicchio, C., J. Appl. Sci., 2022, vol. 12, Article ID: 7779. https://doi.org/10.3390/app12157779
Alamdar, S.S., Foroughifar, N., Shahi, M., and Balali, E., J. Polycycl. Aromat. Compd., 2022, vol. 43, pp. 4354–4370. https://doi.org/10.1080/10406638.2022.2089700
Elkolli, M., Chafai, N., Chafaa, S., Kadi, I., Bensuoici, C., and Hellal, A., J. Mol. Strut., 2022, vol. 1268, Artilce ID: 133701. https://doi.org/10.1016/j.molstruc.2022.133701
Amitha, G.S., Rajan, V.K., Amritha, B., Murraleedharan, K., and Vasudevan, S., J. Photochem. Photobiol. A: Chem., 2019, vol. 382, Article ID: 111904. https://doi.org/10.1016/j.photochem.2019.111904
Cardellichio, C., Ciccarella, G., Naso, F., Perna, F., and Totorella, P., Tetrahedron, 1999, vol. 55, pp. 14685–14692. https://doi.org/10.1016/S0040-4020(99)00914-X
Olyaei, A., Zarnegar, M., Sadeghpour, M., and Rezaei, M., Lett. Org. Chem., 2012, vol. 9, pp. 451–456. https://doi.org/10.2174/157017812801322499
Saidi, M.R., Azizi, N., and Jamal, M.R.N., Tetrahedron. Lett., 2001, vol. 82, pp. 8111–8113. https://doi.org/10.1016/S0040-4039(01)01732-4
Zolfigol, M.A., Ayazi-Nasrabadi, R., Baghery, S., Khakyazadeh, V., and Azizian, S., J. Mol. Catal. A: Chem., 2016, vol. 418–419, pp. 54–67. https://doi.org/10.1016/j.molcata.2016.03.027
Shwetha, N.D., Onkar, A.L., Ramana, M.M.V., and Shrimant, V.R., Russ. J. Bioorg. Chem., 2021, vol. 47, pp. 874–881. https://doi.org/10.1134/S1068162021040075
Ryhkov, F.V., Elison, M., Ryzhkova, Y.E., Vereshchagin, A.N., Fakhrutdinov, A.N., and Egorov, M.P., Chem. Heterocycl. Comp., 2021, vol. 57, pp. 672–678.
Rashid, U., Ahmad, W., Hassan, S.F., Quresh, N.A., Niaz, B., Muhammad, B. Imdad, S., and Sajid, M., Bioorg. Med. Chem. Lett., 2016, vol. 1, pp. 5749–5753. https://doi.org/10.1016/j.bmcl.2016.10.051
Abed, R.R., Mohammed, A.S., and Ahmed, F., Res. J. Pharm. Technol., 2021, vol. 14, pp. 4963–4968. https://doi.org/10.52711/0974-360X.2021.00863
Raauf, A.M.R., Al-Smaism, R.F., Thejeel, K.A., and Rasheed, H.A.M., Nat. Prod., 2019, vol. 33, pp. 1277–1283. https://doi.org/10.1080/14786419.2018.1470516
Nagaraja, O., Bodke, Y.D., Pushpavathi, I., and Ravi Kumar, S., Heliyon, 2020, vol. 6, Article ID: e044245. https://doi.org/10.1016/j.heliyon.2020.e04245
Nithya, P. and Madhavi, C., J. Taibah Univ. SCI, 2017, vol. 11, pp. 40–45. https://doi.org/10.1016/j.jtusci.2014.11.007
Vikram, V., Penumutchu, S.R., Vanakayala, R., Thangudu, S., Amperayani, K.R., and Parimi, U., J. Chem. Sci., 2020, vol. 132, Article ID: 126. https://doi.org/10.1007/s12039-020-01834-W
Kim. J.S., J. Food Sci., 2005, vol. 70, pp. C208–C213. https://doi.org/10.1111/j.1365-2621.2005.tb07127.x
Escobedo, A.A., Topal, B., Kunze, M.B.A., Aranda, J., Chiesa, G., Mungianu, D., Seisdedos, G.B., Eftekharzadeh, B., Gairi, M., Pierattelli, R., Felli, I. C., Diercks, T., Millet, O., García, J., Orozco, M., Crehuet, R., Lindorff-Larsen, K., and Salvatella, X., Nat. Commun., 2019, vol. 10, Article ID: 2034. https://doi.org/10.1038/s41467-019-09923-2
Quiñonero, D., Frontera, A., Garau, C., Ballester, P., Costa, A., and Deya, P.M.J., Chem. Phys. Chem., 2006, vol. 7, pp. 2487–2491. https://doi.org/10.1002/cphc.200600343
Pirkle, W.H. and Liu, Y., J. Chromatogr. A, 1996, vol. 749, pp. 19–24. https://doi.org/10.1016/0021-9673(96)00467-0
Poornima, C.S. and Dean, P.M., J. Comput. Aided Mol. Des., 1995, vol. 9, pp. 500–512. https://doi.org/10.1007/BF00124321
Rahydu, S.V., Karmakar, D., and Kumar, P., Chem. Data Collect., 2021, vol. 33, Article ID: 100709. https://doi.org/10.1016/j.cdc.2021.100709
Blois, M.S., Nature, 1958, vol. 181, pp. 1199–1200. https://doi.org/10.1038/1811199a0
Varpe, B.D. and Jadhav, S.B., Russ. J. Bioorg. Chem., 2022, vol. 48, pp. 372–379. https://doi.org/10.1134/S1068162022020030
Lakshmithendral, K., Saravanan, K., Elacheran, R., Archana, K., Manikandan, N., Arjun, H.A., Ramanathan, M., Lokanath, N.K., and Kabilan, S., Eur. J. Med. Chem., 2019, vol. 168, pp. 1–10. https://doi.org/10.1016/j.ejmech.2019.02.033
Elancheran, R., Saravanan, K., and Choudhury, B., Med. Chem. Res., 2016, vol. 25, pp. 539–552. https://doi.org/10.1007/s00044-016-1504-3
Nagaraja, O., Bodke, Y.D., Kenchappa, R., and Kumar, S.R., Chem. Data Collect., 2021, vol. 33, p. 100713. https://doi.org/10.1016/j.cdc2020100369
Venkatesh, T., Bodke, Y.D., Nagaraj, K., and Ravikumar, S., J. Med. Chem., 2018, vol. 8, pp. 1–7. https://doi.org/10.4172/2161.0444.100488
Arjun, H.A., Rajan, R.K., Elacheran, R., Ramanathan, M., Bhattacharjee, A., and Kabilan, S., Chem. Data Collect., 2020, vol. 26, Article ID: 100350. https://doi.org/10.1016/j.cdc.2020.100350
Pollastri, M.P., Curr. Protoc. Pharmacol., 2010, vol. 49, pp. 9.12.1–9.12.8. https://doi.org/10.1002/0471141755.ph0912s49
Hospital, A., Goni, J.R., Orozco, M., and Gelpi, J., J. Adv. Appl. Bioinform. Chem., 2015, vol. 8, pp. 37–47. https://doi.org/10.2147/AABC.S70333
ACKNOWLEDGMENTS
The authors would like to thank the Chairman of Department of Industrial Chemistry at Kuvempu University in Shankaraghatta for providing laboratory facilities, as well as the University of Mysore and Mangalore for providing spectral data.
Funding
This work was supported by Sc/St cell Kuvempu University, Shankaraghatta, Shimoga, and no, additional grants were obtained.
Author information
Authors and Affiliations
Contributions
All authors made equal contributions to the writing of the article.
Corresponding author
Ethics declarations
This article does not contain any studies involving patients or animals as test objects.
Informed consent was not required for this article. No conflict of interest was declared by the authors.
Additional information
Publisher's Note. Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Pavithra, Pushpavathi, I., Pasha, K.M.M. et al. Synthesis, Biological Evaluation, Molecular Docking, and Molecular Dynamic Simulation Studies of Some New 5-(3,4,5-Trimethoxybenzyl)pyrimidine-2,4-diamine (Trimethoprim) Derivatives via Modified Mannich-Type Reaction. Russ J Bioorg Chem 50, 485–499 (2024). https://doi.org/10.1134/S1068162024020079
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162024020079