Abstract—
Fifteen protein conjugates of penicillins and cephalosporins containing amino- and/or carboxylic groups in the initial structures have been synthesized in the reactions with human serum albumin or ovalbumin using 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride (EDC) or a combination of EDC and N-hydroxysulfosuccinimide at various ratios of the base reagents. A comparative study of conjugates composition and properties has been carried out by UV spectroscopy, mass spectrometry and a ligand-receptor assay. It was shown that the antibiotic residue content of the macromolecules obtained varied from 1 to 22, the beta-lactam cycle remained intact assuring specific interactions of the conjugates with a penicillin-binding protein. In two developed models of receptor bioanalytic systems, an ampicillin conjugate onto a solid phase binds to penicillin-binding protein complexed with a monoclonal antibody, which was detected by an immunoenzyme label in microplate wells or gold nanoparticles on test strips. Conjugated ampicillin binding to the receptor was competitively inhibited by beta-lactam antibiotics added to the liquid phase, and analytical sensitivities relative to penicillin G were 0.05 and 1 ng/mL for microplate and receptor chromatographic systems, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Beta-lactam antibiotics are one of the most widely used classes of antimicrobial drugs in medicine and veterinary chemotherapy [1, 2]. Therefore, accurate, simple and rapid methods for their determination are in demand and are relevant for monitoring the biosafety of food of animal origin [3–6], assessing the level of beta-lactams in the blood in order to prescribe an adequate dose to patients in emergency conditions [6–9], as well as for monitoring content in environmental objects [10, 11]. An immunoassay based on the antigen–antibody reaction has been recognized as an effective method of screening control of various antibiotics [3, 12–15]. However, in the case of beta-lactam antibiotics, a ligand-receptor interaction with the participation of a specific penicillin-binding protein (PBP), which recognizes an essential structural element of these medicinal substances, the beta-lactam ring, presents a unique opportunity for the development of bioassay methods. PBP in the bacterial membrane plays a key role in the antibacterial action of beta-lactams, forming a complex with them with subsequent chemical modification of the serine residue in the active center and the resulting loss of PBP transpeptidase function in the biosynthesis of peptidoglycans of the bacterial cell wall, which leads to the death of the microorganism [16–18]. The advantage of using the receptor in bioassay, in contrast to most antibodies, is the ability to recognize and bind a whole group of beta-lactams, while interaction occurs only with the active forms of these antibiotics, which have a whole beta-lactam ring. Receptor assay test systems are a new and promising tool; in comparison with immunoassay, they are characterized by increased analytical sensitivity and much wider group specificity.
Chemically modified proteins and derivatives of low-molecular-weight organic biomolecules (haptens) are the main reagents for creating immuno- and receptor-analytical systems. At the same time, a highly effective strategy for modifying compounds should ensure the production of biologically active and stable hapten-protein conjugates be distinguished by simplicity and reproducibility. In our work, to obtain protein conjugates of beta-lactam antibiotics, approaches to bioconjugation were chosen, taking into account the presence of reactive groups in the starting compounds and the need to preserve the structure of the beta-lactam ring – the main site of antibiotic recognition by the receptor protein. In this case, both amino groups and carboxyl groups of penicillins and cephalosporins, the most important in practical terms subclasses of beta-lactams, were involved.
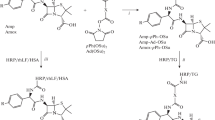
The aminopenicillin molecules are based on the structure of 6-aminopenicillanic acid, which includes condensed thiazolidine and beta-lactam rings (Fig. 1). Aminated cephalosporins are derivatives of 7-aminocephalosporanic and 7-aminodesacetoxycephalosporanic acids containing condensed beta-lactam and dihydrothiazine rings (Fig. 1). For the manifestation of biological activity, the integrity and, accordingly, the reactivity of the beta-lactam cycle, which is very unstable in acidic and alkaline media, and can also be destroyed during chemical modification, are of great importance.
It is not clear from the literature how the chemical nature of the conjugation site in the beta-lactam molecule and the method of its covalent attachment to the protein can affect the behavior of the antibiotic conjugate on the solid phase and the interaction with the receptor protein in the bioanalytical system. Protein conjugates of beta-lactam antibiotics used as immunogens to obtain antibodies and conjugates with horseradish peroxidase for immunoanalytical test systems have been described [13–15, 19, 20]. These conjugates were synthesized using various linkers or the linkerless carbodiimide method. However, the literature lacks quantitative characteristics of antibiotic attachment to a protein in terms of the hapten content in the described conjugates and information on the dependence of this parameter on the molar ratio of reagents, as well as data on the effect of the site of modification of the antibiotic molecule on the conjugation stoichiometry. The relationship between the analytical parameters of the receptor assay test systems and the properties of solid-phase protein conjugates of beta-lactams has not been fully studied.
The aim of this work is to conduct a comprehensive comparative study of methods for direct (“linker-free”) conjugation of penicillins and cephalosporins with inert proteins and to study the dependence of the binding characteristics of conjugates with penicillin-binding protein on their general structure. This knowledge and the obtained reagents are useful in the development of highly sensitive microplate and chromatographic receptor assay systems with a broad group specificity for beta-lactam antibiotics in food.
RESULTS AND DISCUSSION
Synthesis of protein conjugates of beta-lactam antibiotics. To obtain protein conjugates of beta-lactams, bioconjugation methods were chosen taking into account the reactive groups present in the original antibiotics. It was also taken into account that the beta-lactam ring, which is the main site of antibiotic recognition by the receptor protein, should not be destroyed during the synthesis of conjugates. The integrity of the beta-lactam ring was confirmed spectrophotometrically by the absence of absorption peaks at 320–330 nm [21]. The presence of available carboxyl and amino groups in the initial molecules determined the choice of methods using water-soluble carbodiimide and the preparation of activated esters. These synthetic approaches make it possible to carry out the reaction of formation of a strong amide bond between the components of the conjugate in aqueous media (sometimes with the addition of an organic solvent), in which many small biomolecules are sufficiently soluble and proteins can retain their native structure. Carbodiimide (EDC), which has proven itself well in the linker-free synthesis of bioconjugates, forms an intermediate product that is unstable in aqueous solutions with the carboxyl group; therefore, this reagent is often used in combination with N-hydroxysuccinimide or its sulfo-derivative (sNHS) to produce an ester effective in the N-acylation reaction.
Since nearly all proteins contain amino groups, their modification is especially often carried out by means of activated esters of small organic molecules. All beta-lactams are characterized by the presence of a carboxyl group, which, however, is located near the beta-lactam ring. Therefore, we chose such penicillins and cephalosporins (ampicillin, amoxicillin, and cephalexin), which also contain an amino group in the side chain, which makes it possible to spatially separate the functionally important beta-lactam cycle and the amide bond of the antibiotic with the protein in the conjugate. In addition, in contrast to natural penicillin G, synthetic aminopenicillins are quite stable in aqueous solutions at neutral pH, which avoids cleavage of the beta-lactam ring in the conjugation reaction. From cephalosporins, cephalexin was taken, which is very similar in structure to aminopenicillins, but differs in the structure of the cycle condensed with the beta-lactam ring (Fig. 1).
In our work, protein conjugates of aminopenicillins and cephalosporins were synthesized by covalently attaching the studied beta-lactams to human serum albumin (HSA) and ovalbumin molecules by carbodiimide condensation with EDC and in the reaction of activated esters using the EDC/sNHS. In the first case, conjugation occurs mainly at the amino groups of the antibiotic, although reactions involving its carboxyl groups are also possible, and the cross-linking of protein molecules is most likely inhibited due to competition from the amino derivatives of beta-lactams, which have a higher mobility in solution. In variants of synthesis with EDC/sNHS, by changing the order of addition of reagents, the carboxyl group of the antibiotic or protein was activated and then acylated with the obtained activated esters NH2-groups of another component of the conjugation reaction. At the same time, the protein/antibiotic molar ratios ranged from 1 : 10 to 1 : 150.
According to MALDI-TOF mass spectrometry data, the amount of residues attached to proteins (HSA and ovalbumin) of the antibiotic varied from 1 to 22. It was shown that with the same molar ratio of reagents, the amount of attached residues of beta-lactams to HSA depends on the chosen conjugation technique (Table 1). In one-step conjugation with EDC, the addition of 7, 20, and 22 ampicillin residues to one protein molecule was observed at, respectively, 10-, 50- and 150-fold molar excess of this antibiotic. At the same time, in the reaction of activated esters at the same ratios of reagents, other amounts of attached hapten were obtained: upon acylation of the amino groups of the protein molecule – 1, 5 and 6, and upon conjugation at the amino groups of ampicillin – 5, 12 and 19. It is seen that in the case of carbodiimide condensation in comparison with the use of two reagents EDC/sNHS the maximum attachment occurs already with a 50-fold excess of the antibiotic. When the carboxyl groups of beta-lactam are activated, no more than 5–6 hapten residues are attached even with a large molar excess, and in the case of a 10-fold excess of ampicillin, we obtain a conjugate with only an equimolar protein–hapten ratio. Table 1 does not include conjugates of HSA with ceftiofur (C13, synthesized by the method with EDC/sNHS), cefapirin and cefoperazone (C14 and C15, obtained using EDC), which contained, respectively, 5, 12, and 2 antibiotic residues per protein molecule.
Note that, upon conjugation at the amino groups of hapten, in the case of the reaction of activated esters, a consistent and most controlled increase in the amount of attached ampicillin residues is observed according to an increase in its molar load in the reaction mixture.
Spectral characteristics of conjugates. The spectral characteristics of some of the synthesized conjugates (C6, C11, and C12) are shown in Fig. 2. Intact HSA was characterized by the maximum absorption spectrum at 278 nm, and ovalbumin, at 279 nm. The molar extinction coefficient at the absorption maximum for HSA was 3.5 × 104 M–1 cm–1, and for ovalbumin, 3.2 × 104 M–1 cm–1. The shape of the absorption spectra of the conjugates synthesized using various methods (data not shown) was the same as in the spectrum of the initial protein, but a shift in the absorption maximum and a hyperchromic effect were observed, depending on the amount of attached hapten. Thus, the absorption spectra of equimolar conjugates had characteristics that were almost identical to the absorption spectra of unmodified proteins. When more than 1–2 residues of ampicillin, cephalexin or amoxicillin are attached to protein molecules, the absorption spectrum maximum shifts to short wavelengths by 4–6 nm for conjugates with ampicillin and amoxicillin and by 6–8 nm for conjugates with cephalexin. At the same time, there are no additional peaks and shoulders in the bands of the spectra of the conjugates, which indicates the preservation of the intact beta-lactam ring of antibiotics in the conjugates. Thus, absorption spectroscopy proved to be an effective tool for quantitative and qualitative control of the structure of protein conjugates of beta-lactams intended for bio-assay systems.
Receptor assay, binding to receptor protein of beta-lactams and bioanalytical properties of conjugates. The effect of protein conjugation on the binding activity of beta-lactams with respect to PBP has been studied, the bioanalytical properties of the resulting conjugates have been established, and the constructions of model systems for receptor assay in a microplate version and on chromatographic test strips have been developed.
We take a research bioanalytical system as a model as an intermediate object at the generally accepted stage of creating a validated practical system for testing not only standard but also unknown samples prepared from food samples [22]. Compared to immunoassay, receptor assay in laboratory screening studies of the content of beta-lactams in various food matrices has the following advantages: higher sensitivity, wide group specificity, and the ability to detect reactive forms of antibiotics.
In the microplate format of the receptor assay, synthesized conjugates of beta-lactam antibiotics were immobilized on the solid phase, and the main component of the liquid phase was PBP as part of a specific complex with a monoclonal antibody (MAb) to it, which served only as a biological label and did not affect the activity of the ligand-binding center of the receptor protein. In the heterophase system, PBP was distributed between the antibiotic on the solid phase and its competitor in solution as part of “standards” with increasing concentrations of beta-lactam. Detection of bound PBP was carried out using antispecies antibodies (goat immunoglobulins against mouse immunoglobulins) conjugated with horseradish peroxidase.
Comparison of conjugated beta-lactams was carried out according to the parameters of receptor binding, parameters of the calibration curve and analytical characteristics of the test system (Table 2). At the same time, the binding activity of the conjugates was assessed in the absence of competing beta-lactam in solution (B0, a.u.) and parameters of competitive inhibition of the interaction of PBP with a solid-phase antibiotic with increasing concentrations (0.03–900 ng/mL) of penicillin G, ampicillin, and cephalexin. In addition, the signal-to-background difference (ΔВ), the ratio of the optical density values Bi/B0 for standards containing penicillin G (0.05 ng/mL), ampicillin (0.05 ng/mL) and cephalexin (3 ng/mL), the sensitivity of the assay (IC50, ng/mL) and repeatability (CV, %). The results of the study are presented in Table 2.
According to the data obtained, equimolar conjugates bind to the receptor protein more weakly than other conjugates, and for the conjugate with ampicillin B0 it was only 0.52 a.u. In conjugates with ampicillin and amoxicillin at a protein/hapten molar ratio of 1 : (5–7) (C1, C4, C5), the B0 value amounted to 1.17–1.28 a.u., and with a content of 1 : 12 (C6, C12), the corresponding colorimetric signal was already 1.50 p.u. At maximum amounts of conjugated ampicillin (C2, C7) and cephalexin (C11) with protein, the binding parameter B0 amounted to 1.97–2.12 and 1.15 a.u., respectively. As can be seen from the results, cephalexin conjugated with HSA or ovalbumin is characterized by much lower binding to the receptor compared to ampicillin at the same molar fractions of these beta-lactams in the conjugates (C2 and C11; C1, C4, C5 and C10). This indicates a lower affinity of this cephalosporin for PBP, which is also confirmed by the values of the inhibitory activity of IC50 when using cephalexin as a standard (Table 2).
So, in the ligand-receptor system, including the protein conjugate of beta-lactam and PBP, with enzymatic colorimetric detection of the interaction, the optical response largely depends on the nature and amount of attached antibiotic residues in the conjugate. When choosing a solid-phase ligand for the development of a receptor test system for the determination of beta-lactams, one should take into account the fact that we have established that conjugates containing 12–22 ampicillin or amoxicillin residues have the highest binding to PBP.
When comparing the characteristics of conjugates with identical content of beta-lactams (C1, C4, C5, C10; C6, C12; C2, C7, C11), but synthesized by different methods of direct conjugation, we have shown that in terms of parameters B0, ΔВ and IC50 they have similar characteristics of interaction with the receptor and similar bioanalytical properties. This suggests that the site in the beta-lactam molecule through which it directly binds to the carrier protein is not critical for the subsequent interaction of the conjugated antibiotic with the receptor protein. Thus, the beta-lactam ring remains accessible for the ligand-binding center of PBP upon modification of both the amino group at the periphery of the molecule and the carboxyl group near the beta-lactam group.
When comparing the bioanalytical properties of the conjugates, it can be seen that for all solid-phase conjugated antibiotics in the receptor test system, the graphically calculated sensitivity of the assay (IC50) is in the range of 0.34–0.38 ng/mL for penicillin G solutions in standards, 0.39–0.45 ng/mL for ampicillin, and 50–55 ng/mL for cephalexin (Table 2). These data indicate that an increase in the content of antibiotic residues in the composition of the conjugate with the protein insignificantly affects the position of the calibration plot relative to the concentration axis. This property distinguishes receptor assay from the work of most immunochemical test systems using mono- or polyclonal antibodies. So choose the optimal conjugate for receptor assay that provides the highest colorimetric signal B0, without compromising the sensitivity of assay IC50. Conjugates C13–C15 based on ceftiofur, cefapirin, and cefoperazone exhibited activity against PBP, and the characteristics of their binding fit into the structural and functional relationships described above.
Of the synthesized products, protein conjugates of ampicillin (C3) and cephalexin (C8, C9) turned out to be suitable for immunoassay (data not shown), which will be the subject of subsequent publications.
Ampicillin (K7), cephalexin (K11) conjugates were used to assess the group specificity of the assay of beta-lactams in model receptor-enzyme test systems of a microplate design and calibrators—solutions with precise concentrations of individual antibiotics of the beta-lactam group—most commonly used in clinical practice and veterinary medicine. The results of the study are presented in Table 3. It was found that the binding activity of beta-ligands with respect to the receptor protein decreases in the following order: penicillin G > ampicillin > amoxicillin > cefoperazone > cefapirin > ceftiofur > cephalexin. It was also shown that the specificity of the assay does not depend on the nature (penicillin or cephalosporin) of the solid phase ligand. Calibration plots obtained for the tested beta-lactams were characterized by IC50 in the range of 0.35–0.46 ng/mL for penicillins and 0.72–55 ng/mL for cephalosporins, and the cross-reactivity with respect to penicillin G was 82.6–86.4% for penicillins and 0.7–48.6% for cephalosporins. The individual working ranges of reliably determined concentrations of various beta-lactams were within wide limits: 0.05–8.0 ng/mL for penicillins and 0.05–600 ng/mL for cephalosporins.
Comparing the work of C2 and C7 conjugates with practically the same high content of ampicillin attached through its amino group in receptor-enzyme systems, we found that in the case of a two-stage synthesis procedure (C7) the conjugate has slightly better bioanalytical characteristics (B0 and IC50). Therefore, the C7 conjugate was recognized as the optimal ligand for the microplate solid phase, and in subsequent work it was used to immobilize chromatographic test strips on a nitrocellulose membrane.
Expanding our earlier formulation, we note that the analytical characteristics of the system of heterophase receptor–enzyme assay, including the protein conjugate of beta-lactam on the solid phase, are largely determined by the content and nature of the antibiotic in the conjugate and to a lesser extent depend on the site of attachment in the hapten, and also the direct conjugation method.
As a result of experiments to optimize the conditions of ligand-receptor interaction in a microplate system (concentration of C7 conjugate on the solid phase, PBP and ampicillin in solution, pH and ionic strength of the medium, temperature), analytical characteristics were obtained, which are expressed by the calibration curve shown in Fig. 3. The working range of the assay is 0.05–8.0 ng/mL at IC50 = 0.42 ± 0.05 ng/mL (n = 5) and analytical sensitivity 0.05 ng/mL in relation to ampicillin, which exceeds the values presented in the literature [13, 14, 19]. Thus, an immunosensor for fluorescence assay of beta-lactam antibiotics in the work of Benito-Peña et al. [13] was characterized by the following parameters: IC50 = 30 ng/mL and a detection limit of 2.4 ng/mL with a dynamic range of assay 6–191 ng/mL. The limit of detection of ampicillin in a buffered solution in a biosensor using MAb, presented by Cliquet et al. [14], did not exceed 46 ng/mL. Developed by Zeng et al. [19], a test system for the determination of beta-lactams using PBP (a recombinant analog of the natural PBP receptor 2x from Streptococcus pneumoniae R6) allows the determination of ampicillin and penicillin G in the range of 1–16 ng/mL and is characterized by a detection limit for these beta-lactams of 0.75 and 1.22 ng/mL, respectively.
The design of a test system with high sensitivity indicators developed in this work can become a prototype of a set of reagents for the receptor–enzyme assay of beta-lactams in food products of animal origin.
Receptor chromatographic assay. Currently, express methods for the detection of small biomolecules and protein markers on chromatographic test strips are widely used due to the speed and simplicity of execution, high productivity and the possibility of performing assay outside the laboratory without complicated equipment. Such an assay is an effective tool for the qualitative and quantitative determination of antibiotics in food [4, 23].
We have studied the interaction of the synthesized protein conjugate of ampicillin, free beta-lactams and PBP in the developed receptor chromatographic system on a test strip, which is a multimembrane composite. Ampicillin C7 conjugate was immobilized on a working nitrocellulose membrane in the analytical zone, which showed the best results in the microplate system, and the control zone was formed by the adsorption of antispecies antibodies (goat immunoglobulins against mouse immunoglobulins). In the mobile phase, the detecting reagent was PBP, immunochemically immobilized through mouse MAb to PBP, adsorbed on gold nanoparticles, which, in turn, served as a colorimetric label. During the assay, the test strip with a sample membrane was immersed in a solution containing a detection complex and test standard samples with increasing concentrations of beta-lactams (penicillin G, ampicillin, amoxicillin, or cephalexin). When the solution moved along the strip to the absorbent upper membrane, the solid-phase conjugate C7 interacted with the labeled PBP in the analytical zone, and then the adsorbed antispecies antibodies bound with MAb in the detection complex in the control zone. The analytical process consists of one stage and takes 10 minutes. Evaluation of the results consists in visual control of the presence or absence of staining in the analytical and control zones of the test strip (Fig. 4).
Interactions of the MAb-PBP complex labeled with gold nanoparticles with the ampicillin C7 conjugate in the analytical zone and antispecies antibodies in the control zone of the test strip at increasing concentrations of beta-lactams in the mobile phase of the model system of receptor chromatographic assay: (a) ampicillin at a concentration of 0 (1), 0.25 (2), 0.5 (3), 1.0 (4), 2.0 (5), 3.0 (6) and 5.0 ng/mL (7); (b) penicillin G at a concentration of 0 (1), 0.25 (2), 0.5 (3), 1.0 (4), 2.0 (5), 3.0 (6) and 5.0 ng/mL (7); (c) amoxicillin at concentrations of 0 (1), 0.5 (2), 1.0 (3), 2.0 (4) and 5.0 ng/mL (5); (d) cefoperazone at concentrations of 0 (1), 1.0 (2), 2.0 (3), 4.0 (4) and 8.0 ng/mL (5); (e) cephalexin at a concentration of 0 (1), 100 (2), 200 (3) and 400 ng/mL (4).
Various beta-lactam antibiotics as competitive inhibitors of the binding of C7 to labeled PBP in standard solutions with concentrations of 0.25–5.0 ng/mL for penicillins and 1–400 ng/mL for cephalosporins reduced the color intensity of the analytical band, which made it possible to estimate the limits of their detection (Table 4). It has been shown that the threshold of visual detection of ampicillin, penicillin G, and amoxicillin is 1–2 ng/mL, if the influence of the food matrix on the determination results is not considered at this stage. Thus, the developed receptor chromatographic system allows the detection of the listed beta-lactams at concentrations even lower than the maximum residues level (MRL) of these veterinary substances in food [24, 25].
EXPERIMENTAL
Reagents and materials. In the experimental work, we used 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC), N-hydroxysulfosuccinimide (sNHS), 2- (N-morpholino) ethanesulfonic acid (MES), bovine serum albumin (BSA), 30% aqueous solution of H2O2, 3,3',5,5'-tetramethylbenzidine (TMB), thimerosal (Sigma-Aldrich, United States); Zeba columns for chromatography under the influence of gravitational forces for desalting (Thermo Fisher Scientific, United States); dimethyl sulfoxide (Applichem, Germany); sodium chloride, Tween-20, citric acid monohydrate (Merck, Germany); sucrose (Riedel-de-Haën, Germany); columns with Sephadex G-25 (GE Healthcare, United States). Reagents of domestic and Russian manufacturers: sodium phosphate disubstituted 12-water, sodium phosphate monosubstituted 2-water, sodium hydroxide, hydrochloric acid, sulfuric acid, glycerin had a classification not lower than analytical grade. HSA (5% solution) was provided by the Republican Scientific and Practical Center of Transfusiology and Medical Biotechnology (Belarus), goat antibodies against mouse immunoglobulins were obtained from the Unitary Enterprise Pilot Production of the Institute of Bioorganic Chemistry, National Academy of Sciences of Belarus. The conjugate of these antibodies with highly purified (Rz ≥ 3.0) peroxidase from horseradish roots (Dia-m, Russia) was synthesized by oxidation of carbohydrate chains of the enzyme with sodium periodate, addition of immunoglobulin to form a Schiff base, and stabilization of the conjugate by reduction of the double bond with sodium borohydride. Dry powders of highly purified antibiotics: penicillin G, ampicillin, amoxicillin, cephalexin, cefoperazone, ceftiofur, cefapirin were purchased from Sigma-Aldrich, United States. PBP (a recombinant analog of the natural receptor PBP2x from Streptococcus pneumoniae R6) and monoclonal antibodies to it were purchased from Glory Sciences Co., Ltd, China (www.glorybios.com).
For the preparation of all solutions, deionized water with a specific electrical resistance of 17–18 MΩ cm was used obtained in the modular Arium® pro VF water purification system (Sartorius, Germany).
For the receptor–enzyme assay, demountable polystyrene 96-well microplates (OOO Hema-Medica, Russia) were used as solid-phase carriers. Systems for chromatographic assay on test strips designed using a set of membranes from the MDI Easypack (Advanced Microdevices, India).
Obtaining protein conjugates of beta-lactams. In the three methods described below, beta-lactam-protein conjugates were synthesized in the presence of EDC in one step or using EDC/sNHS in two steps in the variants of the initial activation of the antibiotic or protein carboxyls and, accordingly, the attachment of the beta-lactam through the carboxyl or amino group. As a carrier protein, HSA was used in the cases of ampicillin and amoxicillin, or HSA and ovalbumin in the case of cephalexin.
Reaction of carbodiimide condensation in the presence of EDC. The HSA preparation was dissolved in 0.01-M MES (pH 6.0) with 0.15 M NaCl. To solutions containing 15 mg (225 nmol), 3 mg (45 nmol), or 1 mg (15 nmol) HSA, 0.7 mg (3.7 μmol) EDC and 0.8 mg (2.3 μmol) ampicillin in 0.01-M MES (pH 6.0) were added, which corresponded to 10-, 50- or 150‑fold molar excess of the antibiotic in relation to the protein. The conjugation reaction was carried out for 18 h at 4°C.
Bioconjugation by activating antibiotic carboxyl groups using EDC/sNHS. The HSA preparation was dissolved in 0.1-M sodium phosphate buffer (PBS), pH 7.4, with 0.15-M NaCl. Antibiotic preparations were dissolved in 0.01-M MES (pH 6.0) with 0.15-M NaCl. To three antibiotic solutions containing 0.8 mg (2.3 μmol) ampicillin each, 0.8 mg (3.7 μmol) sNHS and 0.7 mg (3.7 μmol) EDC were added, incubated for 15–20 min at 25°C. Then, protein solutions were added containing 15 mg (225 nmol), 3 mg (45 nmol), or 1 mg (15 nmol) HSA, which corresponded to a 10-, 50-, or 150-fold molar excess of the antibiotic in the reaction mixture. The conjugation reaction was carried out for 18 h at 4°C.
Conjugation due to protein carboxyl groups activated by EDC/sNHS. The HSA preparation was dissolved in 0.01-M MES (pH 6.0) with 0.15-M NaCl. To solutions containing 15 mg (225 nmol), 3 mg (45 nmol), or 1 mg (15 nmol) HSA, 0.8 mg (3.7 μmol) sNHS and 0.7 mg (3.7 μmol) EDC were added, incubated for 15–20 min at 25°C. Then, 0.8 mg (2.3 μmol) of ampicillin in 0.1-M PBS (pH 7.0) with 0.15-M NaCl was added to the protein solutions, which corresponded to a 10-, 50-, or 150-fold molar excess of the antibiotic relative to the protein. The conjugation reaction was carried out for 18 h at 4°C.
Using similar approaches, conjugates of cephalexin with HSA and ovalbumin were prepared (10-, 50-, 150-fold molar excess of the antibiotic in the reactions). Amoxicillin-HSA conjugate was synthesized via NH2-group of the antibiotic using its 150-fold molar excess.
All conjugates obtained were purified by gravity chromatography using Zeba columns equilibrated with 0.02 M PBS (pH 7.2) containing 0.15 M NaCl. Purified conjugates were diluted 2-fold with glycerol and stored at –20°C.
Spectral measurements. The absorption spectra of solutions of antibiotics and synthesized conjugates were measured in a cuvette with an optical path length of 1 cm using a Tecan Infiniti M200 spectrophotometer (Austria).
Mass spectra. MALDI-TOF mass spectra were recorded on a Microflex LRF instrument (Bruker, Germany). The change in the molecular weight (M) of proteins upon conjugation, ΔM, expressed in Da, was determined as the difference in the molecular weights of the conjugate and unmodified protein. The degree of conjugation (1 mol of antibiotic per 1 mol of protein) was calculated as ΔM/M (antibiotic).
Biospecific binding and competitive inhibition of interactions in receptor-enzyme systems. Protein conjugates of antibiotics were immobilized in the wells of the microplate from 100 μL of a solution with a concentration of 0.1 μg/mL by incubation at 4–8°C for 18 hours. Stabilization was carried out by adding 150 μL of 0.05 M PBS (pH 7.4) containing 0.15-M NaCl, 0.05% Tween-20, 1 mg/mL BSA, 2% sucrose, 0.01% euxil K-100 to all wells, and keeping the plate at 4–8°C for 18 hours. Stock solutions of the studied antibiotics at a concentration of 4 mg/mL were prepared in 0.01-M PBS (pH 7.2), 0.15-M NaCl. Standards (calibration solutions) were prepared in the same buffer at a concentration of 0.03–8.0 ng/mL for penicillin G, amoxicillin, ampicillin, cefoperazone; 0, 0.05–100.0 ng/mL for ceftiofur and cefapirin; 1–900 ng/mL for cephalexin.
During the analysis, 50 μL of calibration solutions with an antibiotic in increasing concentrations and 50 μL of a preliminarily prepared mixture containing PBP and MAb at concentrations of 0.05 and 0.1 μg/mL, respectively, were added to the wells. The system was incubated in the plate at 25°C for 30 min. Then, unreacted components were removed and the plate was washed using a wash solution (0.01-M PBS, pH 7.2, 0.15-M NaCl, 0.05% Tween-20). At the second stage, 100 μL of a solution of the conjugate of antispecies antibodies (goat immunoglobulins against mouse immunoglobulins) with horseradish peroxidase in a titer of 1 : 25 000. After incubation at 25°C for 15 min, the contents of the wells were removed and the plate was washed as described above. The wells were filled with 100 μL of chromogen-substrate solution containing TMB and hydrogen peroxide (0.1-M sodium citrate buffer, pH 4.2, 3-mM H2O2 and 1-mM TMB), and incubated for 15 min at 20–25°C. The enzymatic reaction was stopped by adding 100 μL of 5% H2SO4.
The optical density was measured at 450 nm (OD450) in a Tecan Infinity M200 microplate spectrophotometer (Austria) and the ratio Bi/B0 (%), where B0 is the optical density in the absence of beta-lactam in solution, and Bi is the optical density in the presence of increasing concentrations of antibiotic in solution. Calibration curves of the dependence of competitive binding on the concentration of beta-lactam in calibration standards were plotted in the coordinates: Bi/B0 (%, ordinate is linear) and concentration (ng/mL, abscissa is logarithmic).
The overall sensitivity of the method was assessed by the graphically determined value of IC50, which corresponds to the concentration of the antibiotic in the solution causing 50% inhibition of the binding of the receptor to the solid-phase ligand (decrease in B0 twice). Analytical sensitivity (detection limit, minimum reliably measured concentration) was obtained from the calibration graph as the abscissa of point B0 – 2SD, where SD is the standard deviation. Cross-reactivity (CR) was calculated using the formula: CR (%) = IC50 (ampicillin)/IC50 (another antibiotic) × 100.
Receptor chromatographic assay on test strips. The reagents were applied to the nitrocellulose membrane using an IsoFlow automatic dispenser (Imagene Technology, United States). The analytical zone of the test strip was formed by applying the conjugate of ampicillin C7 from a solution with a concentration of 0.5 mg/mL, and in the control zone, antispecies antibodies (goat immunoglobulins against mouse immunoglobulins) were immobilized from a solution with a concentration of 0.25 mg/mL. The functionalized membranes were dried in air at 20–22°C for at least 20 h and, together with the support for the test sample and the absorbent membrane, were assembled into a multimembrane composite. Test strips were cut 3.5 mm wide using an IndexCutter 1 automatic guillotine cutter (A-Point Technologies, United States).
Gold nanoparticles were prepared by the reduction of chloroauric acid with sodium citrate according to the Frens method, as described by Byzova et al. [26]. The resulting nanoparticles at a concentration of 10 μg/mL selected on the basis of photometric data were adsorptively conjugated with MAb to PBP. To carry out model receptor chromatographic assay of beta-lactams, the MAb-PBP complex labeled with gold nanoparticles was added to the wells of an inert plastic microplate, and beta-lactam (ampicillin, penicillin G, amoxicillin, cefoperazone, or cephalexin) was added at increasing concentrations of 0.25–400 ng/mL. Then, the reagents were kept for 3–5 min, and prepared test strips were placed in the well. Chromatography was performed for 10 min and staining was visually recorded in the analytical and control zones.
All experiments on ligand-receptor binding were performed in at least three repetitions. The data were processed using Microsoft Excel. In the calibration graph (Fig. 3), the error bars represent the standard deviation SD.
CONCLUSIONS
As a result of the study, the methods of direct conjugation of penicillins and cephalosporins with protein macromolecules (HSA and ovalbumin) for receptor assay technologies were characterized. The effect of modifications of penicillins and cephalosporins in the region of the beta-lactam ring and in the side chain on binding to the receptor protein was established, and the spectral characteristics of the synthesized protein conjugates of beta-lactams were determined. Optimal conditions for the bioconjugation of proteins with these substances without preliminary introduction of linker (spacer) groups into the structures of antibiotics have been proposed. The optimal molar ratio of antibiotic and carrier protein in the synthesized conjugate for its effective interaction with PBP has been established. Bioanalytical properties of the conjugates obtained were studied in model systems of ligand-receptor binding. Designs of test systems for receptor assay of beta-lactam antibiotics in a microplate format and in the chromatographic test strips, characterized by a wide group specificity and high analytical sensitivity in relation to penicillins and cephalosporins, have been developed.
In the near future, the model receptor chromatographic test system described in this work for detecting beta-lactams can become a practical analytical tool for express control of food biosafety.
REFERENCES
Sachi, S., Ferdous, J., Sikder, M.H., Azizul, Karim., and Hussani, S.M., J. Adv. Vet. Anim. Res., 2019, vol. 6, pp. 315–332. https://doi.org/10.5455/javar.2019.f350
Landers, T.F., Cohen, B., Wittum, T.E., and Larson, E.L., Public Health Rep., 2012, vol. 127, pp. 4–22. https://doi.org/10.1177/003335491212700103
Kantiani, L., Farre, M., and Barcelo, D., TrAC, Trends Anal. Chem. (Pers. Ed.), 2009, vol. 28, pp. 729–744. https://doi.org/10.1016/j.trac.2009.04.005
Dzantiev, B.B., Byzova, N.A., Urusov, A.E., and Zherdev, A.V., TrAC, Trends Anal. Chem. (Pers. Ed.), 2014, vol. 55, pp. 81–93. https://doi.org/10.1016/j.trac.2013.11.007
Zhang, J., Wang, Z., Wen, K., Liang, X., and Shen, J., Anal. Biochem., 2013, vol. 442, pp. 158–165. https://doi.org/10.1016/j.ab.2013.07.042
Merola, G., Martini, E., Tomassetti, M., and Campanella, L., J. Pharm. Biomed. Anal., 2014, vol. 106, pp. 186–196. https://doi.org/10.1016/j.jpba.2014.08.005
Abdulla, A., Bahmany, S., Wijma, R.A., van der Nagel, B.C.H., and Koch, B.C.P., J. Chromatogr. B Anal. Technol. Biomed. Life Sci., 2017, vol. 1060, pp. 138–143. https://doi.org/10.1016/j.jchromb.2017.06.014
Briscoe, S.E., McWhinney, B.C., Lipman, J., Roberts, J.A., and Ungerer, J.P.J., J. Chromatogr. B Anal. Technol. Biomed. Life Sci., 2012, vol. 907, pp. 178–184. https://doi.org/10.1016/j.jchromb.2012.09.016
Rawson, T.M., Sharma, S., Georgiou, P., Holmes, A., Cass, A., and O’Hare, D., Electrochem. Commun., 2016, vol. 82, pp. 1–5. https://doi.org/10.1016/j.elecom.2017.07.011
Merola, G., Martini, E., Tomassetti, M., and Campanella, L., Sens. Actuators, 2014, vol. 199, pp. 301–313. https://doi.org/10.1016/j.snb.2014.03.083
Hrioua, A., Loudiki, A., Farahi, A., Bakasse, M., Lahrich, S., Saqrane, S., and Mhammedi, M.A.El., Bioelectrochemistry, 2021, vol. 137, p. 107687. https://doi.org/10.1016/j.bioelechem.2020.107687
Byzova, N.A., Zvereva, E.A., Zherdev, A.V., and Dzantiev, B.B., Appl. Biochem. Microbiol., 2011, vol. 47, pp. 627–634. https://doi.org/10.1134/S0003683811060032
Benito-Pena, E., Moreno-Bondi, M.C., Orellana, G., Maquieira, A., and van Amerongen, A., J. Agric. Food Chem., 2005, vol. 53, pp. 6635–6642. https://doi.org/10.1021/jf0511502
Cliquet, P., Goddeeris, B.M., Bonroy, K., and Cox, E., Food Agric. Immunol., 2005, vol. 16, pp. 101–115. https://doi.org/10.1080/09540100500139239
Cliquet, P., Cox, E., Van Dorpe, C., Schacht, E., and Goddeeris, B.M., J. Agric. Food Chem., 2001, vol. 49, pp. 3349–3355. https://doi.org/10.1021/jf001428k
Macheboeuf, P., Contreras-Martel, C., Job, V., Dideberg, O., and Dessen, A., FEMS Microbiol. Rev., 2006, vol. 30, pp. 673–691. https://doi.org/10.1111/j.1574-6976.2006.00024.x
Sauvage, E., Kerff, F., Terrak, M., Ayala, J.A., and Charlier, P., FEMS Microbiol. Rev., 2008, vol. 32, pp. 234–258. https://doi.org/10.1111/j.1574-6976.2008.00105.x
Mbah, A.N. and Isokpehi, R.D., Chemother. Res. Pract., 2013, vol. 2013, Article ID 614670. https://doi.org/10.1155/2013/614670
Zeng, K., Zhang, J., Wang, Y., Wang, Z.H., Zhang, S.X., and Chong, MingW.U., Biomed. Environ. Sci., 2013, vol. 26, pp. 100–109. https://doi.org/10.3967/0895-3988.2013.02.004
Peng, J., Cheng, G., Huang, L., Wang, Y., Hao, H., and Peng, D., Anal. Bioanal. Chem., 2013, vol. 405, pp. 8925–8933. https://doi.org/10.1007/s00216-013-7311-5
Komova, N.S., Berlina, A.N., Zherdev, A.V., and Dzantiev, B.B., Orient. J. Chem., 2020, vol. 36, pp. 21–25. https://doi.org/10.13005/ojc/360103
Vashkevich, I.I., Yastrebova, A.A., Kuprienko, O.S., Serchenya, T.S., Ivan’ko, M.V., Shkinderova, V.O., Pyzhik, I.P., Smolyak, T.M., Meleshchenya, A.V., and Sviridov, O.V., Vest. Nats. Akad. Navuk Belarusi, Ser. Khim. Navuk, 2020, vol. 56, no. 3, pp. 318–332. https://doi.org/10.29235/1561-8331-2020-56-3-318-332
Chen, W., Huang, Z., Hu, S., Peng, J., Liu, D., Xiong, Y., Xu, H., Wei, H., and Weihua, L., J. Dairy Sci., 2019, vol. 102, pp. 1887–1900. https://doi.org/10.3168/jds.2018-15462
The List of Veterinary Medicinal Products (Pharmacologically Active Substances), the Maximum Permissible Levels of Residues of Which May Be Contained in Unprocessed Food Products of Animal Origin, Including Raw Materials, and Methods for Their Determination, Decision of the Board of the Eurasian Economic Commission dated February 13, 2018, no. 28. http://pravo.by/document/?guid=3871&p0=F91800044
European Commission. Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin, Off. J. Eur. Union, 2010, L 15/1. https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-5/ reg_2010_37/reg_2010_37_en.pdf.
Byzova, N.A., Smirnova, N.I., Zherdev, A.V., Eremin, S.A., Shanin, I.A., Lei, H.T., Sun, Y., and Dzantiev, B.B., Talanta, 2013, vol. 119, pp. 125–132. https://doi.org/10.1016/j.talanta.2013.10.054
Funding
The research was carried out on the topic of task 2.3.1 of the state program of scientific research “Chemical processes, reagents and technologies, bioregulators and bioorganic chemistry,” funded by the National Academy of Sciences of Belarus.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare they have no conflict of interest.
No humans or animals were used as research subjects in this study.
Additional information
Abbreviations: CV, coefficient of variation; MAb, monoclonal antibody; PBP, penicillin-binding protein; HSA, human serum albumin; В0, optical density in the absence of competing beta-lactam in the liquid phase; BSA, bovine serum albumin; EDC, 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride; IC50, concentration of antibiotic in solution causing 50% inhibition of receptor binding to solid phase ligand; MES, 2-(N-morpholino)ethanesulfonic acid; PBS, phosphate-buffered saline; sNHS, N-hydroxysulfosuccinimide.
Corresponding autor: phone: +375 (17) 300-57-07.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Serchenya, T.S., Harbachova, I.V. & Sviridov, O.V. Direct Conjugation of Penicillins and Cephalosporins with Proteins for Receptor Assays of Beta-Lactam Antibiotics. Russ J Bioorg Chem 48, 85–95 (2022). https://doi.org/10.1134/S1068162022010125
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162022010125