Abstract
A new approach to aminopenicillin modification and conjugation with proteins was developed using di-N-hydroxysuccinimide esters of dicarboxylic acids as crosslinkers. Acylation of ampicillin (Amp) and amoxicillin (Amox) with di-N-hydroxysuccinimide esters of adipic or terephthalic acids was carried out in an organic solvent. Subsequent conjugation of the resulting aminopenicillin derivatives with proteins was done in an aqueous medium at pH 8.3 to produce immunogenic and enzymatic conjugates of Amp and Amox. The β-lactam cycle of Amp was shown to remain intact after chemical modification and synthesis of linker conjugates. An immunogenic Amp–thyroglobulin conjugate containing an aromatic linker was used for long-term immunization of rabbits, and polyclonal antibodies thus obtained were found to bind Amp, Amox, and penicillin G with extremely high sensitivity. Amp and Amox conjugates with horseradish peroxidase (HRP) were synthesized and characterized in a competitive protein-binding (receptor) assay and a direct competitive enzyme-linked immunosorbent assay (ELISA). Of the model immunoassay systems tested, the best characteristics were observed for heterologous direct ELISA with polyclonal antibodies and the Amp–HRP conjugate that contained an adipic acid fragment as a linker: the Amp sensitivity was 0.03 ng/mL and IC50 = 0.20 ng/mL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
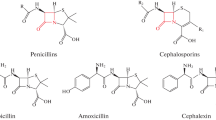
β-Lactam antibiotics, particularly penicillins, are broadly used in veterinary practice. Residual amounts of these compounds may occur in foods of animal origin and enter the human body to substantially affect human health. The antibiotics may provoke serious allergic reactions, alter the intestinal microflora, and cause the development of resistant microbial strains [1]. Maximum residue limits have been established for β-lactam residues found in unprocessed foods of animal origin in the Eurasian Economic Union and the European Union. In particular, the limits of penicillin G, ampicillin (Amp), and amoxicillin (Amox) are 4 μg/kg for milk and 50 μg/kg for meat [2, 3].
To ensure biological safety of foods, various methods have been developed for penicillin detection. Liquid chromatography with mass-selective detection is one of the methods [4–6], but is expensive, labor intensive, and time consuming because sample preparation is difficult and takes a long time. Immunochemical methods provide an alternative, being rapid and relatively inexpensive. Their development and application requires protein reagents (receptors and antibodies) that are capable of highly sensitive penicillin binding, as well as high-molecular-weight derivatives of the antibiotics as haptens: enzyme conjugates, immunogens, and conjugated antigens.
Proteins with high specific affinity for penicillins are possible to classify into two groups. One includes penicillin-binding proteins (PBPs), which perform structural and protective functions in bacteria. In particular, the group includes a high-molecular-weight membrane transpeptidase PBP2x of Streptococcus pneumoniae R6 [7] and the carboxy terminal domain of the β-lactam transmembrane receptor (BlaR-CTD) of Bacillus licheniformis [8]. These proteins recognize the antibiotic structure that contains the β-lactam cycle and bind various β-lactams, including both penicillins and cephalosporins. The broad specificity of bacterial PBPs is their main advantage in the context of their use in receptor analytic systems, but restricts their utility because substantially different maximum residue limits in foods are established for penicillins and cephalosporins [2, 3].
Specific antibodies are the other group of penicillin-binding proteins. Both polyclonal [9–13] and monoclonal [14–16] antibodies have been described for penicillins. Antibodies have also been obtained to recognize the hydrolysis products of β-lactam antibiotics [13, 17]. A penicillin sensitivity of ~1 ng/mL is observed with only few of the enzyme-linked immunosorbent assay (ELISA) systems that utilize the above antibodies [10, 13, 16]. Given the maximum residue limits of β-lactams, this ELISA sensitivity might be insufficient for determining penicillin G, Amp, and Amox in milk diluted by a factor of 5–10. Yet manifold dilution is often the only means to reduce the nonspecific effect that components of the sample exert on the antigen–antibody interaction.
Chromatographic strip tests provide a convenient variant of immunochemical and receptor systems. Immunochromatographic assays for a broad range of β-lactams have been performed using PBP [18, 19] and an Amp-specific monoclonal antibody [20].
Various methods to conjugate β-lactam antibiotics with proteins have been described in the literature. Crosslinkers were used to form an aliphatic or aromatic insert between the protein and antibiotic moieties [7, 9–11, 14, 15, 18]. Compounds that directly covalently link the antibiotic and protein moieties (without a linker) were described [7–10, 12, 14, 15, 19]. A “physiological” conjugation technique was developed taking advantage of the high reactivity of the β-lactam cycle [7, 13, 14]. Each of the methods has its drawbacks. For example, glutaraldehyde is of limited utility because crosslinks are highly likely to arise between protein polypeptide chains and unstable physicochemical properties are probable for the resulting conjugates. Because both carboxyl and amino groups are present in the aminopenicillin structures, water-soluble carbodiimide fails to ensure conjugation via only one of the two groups. Immune reagents synthesized via physiological pathways cannot be employed in receptor protein-based assays, and their use in tests with specific antibodies requires the sample preparation procedures that fully disrupt the β-lactam cycle of an antibiotic in a food sample [13, 17].
The objective of this study was to develop a new method to synthesize aminopenicillin–protein conjugates. The method had to ensure a stable link between the protein and hapten moieties, to exclude the possibility of intra- and intermolecular crosslinks in polypeptide chains, and to induce no damage to the β-lactam ring in penicillins. In addition, we intended to obtain high-affinity anti-Amp antibodies that could be used together with conjugates synthesized by our method to design highly sensitive ELISA systems for detecting penicillins in foods.
RESULTS AND DISCUSSION
Synthesis of aminopenicillin derivatives and their conjugation with proteins. A two-step method was designed to synthesize aminopenicillin conjugates with the use of homobifunctional crosslinkers. The gist is synthesizing N-hydroxysuccinimide esters (OSu esters) of carboxyl aminopenicillin derivatives and isolating them from the reaction mixture to allow long-term storage and subsequent simple and rapid conjugation with proteins.
Di-N-hydroxysuccinimide ester of terephthalic acid (pPh(OSu)2) was used as a crosslinker. To obtain pPh(OSu)2, the respective dichloroanhydride (a methylene chloride solution) was combined with a 3.5-fold excess of N-hydroxysuccinimide (NHS). As a result of the reaction occurring in the presence of a base, the product pPh(OSu)2 precipitated to form a white precipitate. To synthesize di-N-hydroxysuccinimide ester of adipic acid (Ad(OSu)2), diisopropylcarbodiimide was added to an adipic acid–NHS mixture in dioxan. Ad(OSu)2 was precipitated from the reaction mixture with diethyl ether after removing the diisopropyl urea precipitate, which also formed in the reaction. The structures of the synthesized crosslinkers were verified by NMR and mass spectrometry. The 1H-NMR spectrum of pPh(OSu)2 showed signals from protons of the methylene groups of the succinimide moiety (δ 2.92 ppm) and the benzene ring (δ 8.34 ppm); the 1H-NMR spectrum of Ad(OSu)2 showed signals from protons of the methylene groups of the succinidmide moiety (δ 2.81 ppm) and adipic adic (δ 1.68–1.74 and 2.74 ppm). Peaks corresponding to the molecular ions [M + Na]+ and [2M + Na]+ were observed in the mass spectra of the crosslinkers.
A scheme of the synthesis of linker aminopenicillin conjugates is shown in Fig. 1. Table 1 summarizes the structures of the substituent R and linker X and the characteristics of the initial reagents and the resulting antibiotic derivatives. At the first step, an Amp or Amox solution in an organic solvent was added to a 1.1- to 1.2-fold excess of pPh(OSu)2 or Ad(OSu)2. The reaction was deemed to be completed when ninhydrin staining did not detect a spot on a thin-layer chromatography plate. The activated ester Amp-pPh-OSu, Amp-Ad-OSu, or Amox-pPh-OSu was precipitated from the reaction mixture with diethyl ester. Both [M + H]+ protonated molecules and [M + Na]+ molecular ions were observed in mass spectra of the resulting aminopenicillin derivatives (Table 1).
Scheme of aminopenicillin conjugate synthesis. Reaction conditions: (i) DMF, Et3N, 20–25°C, 2.5 h; (ii) 0.1 М NaHCO3 (pH 8.3), 4°C, 16 h; (iii) EDC or EDC/sNHS (pH 6.0), 4°C, 16 h. Formulas of the substituents (R) and linkers (X) are shown in Table 1.
At the second step, weighed quantities of the synthetic aminopenicillin derivatives were dissolved in dimethylformamide (DMF). A 20-fold molar excess of a derivative was added to a horseradish peroxidase (HRP) solution in 0.1 M NaHCO3 (рН 8.3); the volume concentration of the organic solvent was no more than 5% in the final reaction mixture. The mixture was incubated at 4°C for 16 h. Thyroglobulin (TG) modification was carried out in similar conditions, but with a far greater (670-fold) molar excess of Amp-pPh-OSu. The resulting conjugates had the Amp or Amox moiety linked through a six-atom aliphatic bridge or aromatic core to the amino groups of amino acid residues of the protein polypeptide chain. The unbound excess reagents were removed, and the incorporation rates of the antibiotics in conjugates were measured by comparing the mass spectrum between the initial and modified proteins (Table 1). Because the large molecular mass of TG, TG conversion to Amp-pPh-TG was inferred from the substantial increase in light absorption in a range of 250–280 nm by a conjugate solution.
It should be noted that Cliquet et al. [14] have used a similar aromatic insert between Amp and protein moieties. Amp was acylated with OSu ester of 3-maleimidobenzoic acid and added to solutions of bovine serum albumin (BSA) or ovalbumin, which preliminarily underwent two-step modification to include additional sulfhydryl groups. Our method to synthesize aminopenicillin conjugates with the use of di-OSu esters is simpler and faster, proceeds without time-consuming protein modification, and yields conjugates with stable bonds.
The linkerless conjugates Amp-HRP and Amp-rhLF were synthesized according to the same scheme (Fig. 1), by adding water-soluble 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) to an Amp mixture with HRP or recombinant human lactoferrin (rhLF). The Amp conjugate with human serum albumin (HSA) was synthesized as described previously [19], with an intermediate step where an activated Amp ester was obtained as a result of the Amp interaction with N-hydroxysulfosuccinimide in the presence of EDC and then added to a HSA solution. The Amp incorporation rates based on the mass spectra of the conjugates and initial proteins are summarized in Table 1.
Binding of low-molecular-weight derivatives and enzyme conjugates of aminopenicillins with PBP. Penicillins are unstable compounds because the β-lactam ring is labile. To find out whether the β-lactam cycle is destroyed in the conditions of derivative synthesis, the derivatives were tested for binding with a PBP that recognizes the intact β-lactam group. The PBP was previously used in an indirect receptor assay on microplates and a chromatographic receptor assay to detect β-lactam antibiotics [19]. Using the PBP, the stability of the Amp β-lactam ring was checked in the conditions of Amp acylation with pPh(OSu)2 (DMF as an organic solvent, Et3N as a base, a higher temperature) and protein conjugate synthesis (рН 8.3, 4°C, 16 h). Binding activity estimates were calculated for Amp and its derivatives as described in Experimental and summarized in Table 2. The interaction of Amp with PBP was not affected by a short-time heating of the Amp solution in DMF in the presence of Et3N at 50°C, incubation with 0.1 M NaHCO3 (pH 8.3) at 4°C for 16 h, and acylation of the amino group with pPh(OSu)2 (the binding activity was 95–105%, within the variation limits allowable for biological testing results). However, when Amp was incubated in solution at pH 8.3 at 37°C for a period of time longer than the duration of synthesis, Amp binding measured in the receptor assay decreased by 63%. Thus, disruption of the β-lactam cycle did not take place at any step of synthesizing the low-molecular-weight Amp derivative and its protein conjugates.
Binding characteristics of the aminopenicillin–HRP conjugates were compared in the competitive receptor assay. To obtain the A450 colorimetric signal falling in a range of 1.4–1.6 units, the linker conjugates Amp-pPh-HRP, Amp-Ad-HRP, Amox-pPh-HRP were used at 0.5 μg/mL and the linkerless conjugate Amp-HRP was used at 2.5 μg/mL. The linker conjugates were found to similarly interact with PBP in the presence of increasing Amp amounts, while a different binding pattern was observed for the direct Amp-HRP conjugate. The analytic sensitivities of model receptor systems utilizing the linker conjugates or Amp-HRP were, on average, 0.11 and 0.25 ng/mL, respectively. The Amp concentration that inhibited the HRP conjugate binding by 50% (IC50) averaged 0.60 ng/mL in the systems with Amp-pPh-HRP, Amp-Ad-HRP, Amox-pPh-HRP and was 0.76 ng/mL in the system with Amp-HRP (Fig. 2). The difference might be explained by partial degradation of the β-lactam ring during Amp-HRP synthesis. It is also possible that Amp accessibility was lower in the conjugate where Amp is directly linked to a protein.
Immunogenic aminopenicillin conjugates and generation of polyclonal antibodies. The Amp-pPh-TG and Amp-rhLF conjugates were used to immunize two groups of rabbits in order to produce anti-Amp polyclonal antibodies. To evaluate the immunogenicity of the conjugates, the resulting antisera were tested by indirect ELISA, using the linkerless Amp-HSA conjugate with a low Amp incorporation as an agent immobilized on a solid phase, increasing Amp concentrations, and various antiserum dilutions. Bound specific antibodies were detected using enzyme-conjugated anti-rabbit Ig goat antibodies and the HRP-catalyzed color reaction of TMB oxidation in the presence of H2O2. The intensity of the A450 signal depended on the content of anti-Amp antibodies in the antiserum sample and the Amp concentration in the liquid phase of the system. Indirect ELISA parameters established in our study are summarized in Table 3 in comparison with published data [12]. The working titer of antisera obtained by immunization with the Amp-pPh-TG conjugate was approximately three times higher than that of antisera obtained in the case of immunization with Amp-rhLF.
A higher immunogenicity of the former conjugate might be associated with the nature of the carrier protein. It has been found that TG is preferable to use to obtain anti-Amp antibodies as compared with lower-molecular-weight proteins, such as BSA and ovalbumin [14]. We confirmed the earlier finding that an aromatic linker introduced in an immunogen molecule between the Amp and protein carrier moieties does not prevent the production of specific anti-Amp antibodies [14]. Moreover, we showed that antibodies with a high sensitivity to penicillins are produced in response to an immunogen with the above structure.
As has already been observed, only single rabbits in a group produce an intense response to administration of an Amp-containing immunogen [9, 10]. We found additionally that the Amp sensitivity of the resulting antibodies as assessed by indirect ELISA greatly varied not only between the rabbit groups, but also within a group immunized with the same conjugate. The analytical sensitivity of the assay varied from 2 to 20 ng/mL in the case of Amp-rhLF used as an immunogen and from 1 to 1.0 ng/mL in the case of the Amp-pPh-TG conjugate.
It should be noted that antibody obtained in our work showed group specificity to penicillins, making it possible to determine, in a single test, the total content of at least three antibiotics of the penicillin group: Amp, penicillin G, and Amox (other members of the group were not tested). When a conjugate synthesized using glutaraldehyde as a crosslinker was used to immunize rabbits [9, 10], the resulting antibodies had a similar group specificity, but were substantially inferior in sensitivity to the best polyclonal antibodies obtained in our work.
Polyclonal antibodies and peroxidase conjugates in direct ELISA. Immunoanalytical properties of the aminopenicillin–HRP conjugates were studied in model heterophasic systems based on direct interactions with antibodies biospecifically immobilized from the antisera obtained with Amp-pPh-TG and Amp-rhLF. In the case of the antiserum to Amp-pPh-TG (Fig. 3a), the linker conjugates were used at 0.07 μg/mL and the linkerless Amp-HRP conjugate was used at 0.25 μg/mL. The average analytical sensitivity was 0.03 ng/mL with all enzyme conjugates (calculations were performed as described in Experimental). IC50 varied from 0.20 to 0.40 ng/mL, being maximal in the case of the Amp-pPh-HRP conjugate. The binding of this conjugate with antibodies was 40% of its maximum at the end of the calibration curve. At the same time, the Amp-Ad-HRP, Amox-pPh-HRP, and Amp-HRP conjugates were displaced by 2 ng/mL Amp to the extent of 70–80%.
Similar interactions with antibodies present in the antiserum to Amp-rhLF were observed for the linker conjugates Amp-pPh-HRP, Amp-Ad-HRP, and Amox-pPh-HRP (Fig. 3b). The Amp-pPh-HRP enzyme conjugate was used in direct ELISA at 0.3 μg/mL; the Amp-Ad-HRP and Amox-pPh-HRP conjugates were used at 0.5 μg/mL. The sensitivity of the assay was 2.0 ng/mL; IC50 was 17–20 ng/mL. The amp-HRP conjugate, which coincided in general structure with the immunogen, was used at 0.3 μg/mL and formed complexes with antibodies against Amp-rhLF, but was not displaced even with 1 μg/mL Amp.
Thus, direct ELISA with antibodies against Amp-pPh-TG was found to be more sensitive than the indirect assay (Table 3). The results of direct ELISA (Fig. 3) confirmed that antibodies from the antiserum to Amp-pPh-TG were more sensitive to penicillin antibiotics than polyclonal antibodies from the antiserum to Amp-rhLF. In line with observations by Wang et al. [21], we found additionally that better immunoanalytical parameters were ensured by heterologous immunogen and conjugated antigen constructs, which differed in the structure of the hapten used for their synthesis. In the case of the antiserum to Amp-pPh-TG, the effect was observed both when the hapten–protein linker differed between the constructs and when the linker was the same, but the constructs included Amp or Amox.
Using the most sensitive of our model direct ELISA systems, we studied the dynamics of specific polyclonal antibody production and checked the antigen-binding activity (enzyme conjugate binding and IC50) of antibodies from rabbits immunized with Amp-pPh-TG (Fig. 4). An antiserum with better binding characteristics was obtained 33 weeks from the start of immunization, that is, after the tenth immunogen injection. When immunization was continued further, the sensitivity and specificity of antiserum antibodies remained much the same and the specific antibody concentration did not increase. Prolonged immunization has already been reported as a necessary condition for producing penicillin-specific antibodies [10]. There are also data that an antiserum with a high titer, but a low analytical sensitivity (~5.0 ng/mL) is possible to obtain quickly [12].
EXPERIMENTAL
We used ampicillin trihydrate, amoxicillin trihydrate, penicillin G sodium salt, BSA, EDC, sNHS, terephthalic acid dichloroanhydride, adipic acid, diisopropylcarbodiimide, DMF, triethylamine, TMB, complete Freund’s adjuvant, Tween-20, HRP-conjugated antirabbit Ig goat antibodies (Sigma-Aldrich, United States), and HRP (Panreac Quimica SLU, Spain). PBP (a recombinant analog of the natural Streptococcus pneumoniae R6 PBP2x receptor) and anti-PBP monoclonal antibody were purchased from Glory Sciences (China, www.glorybios.com).
TG isolated from extracts of human thyroid gland tissue (surgery material), antimouse Ig sheep antibodies, and antirabbit Ig sheep antibodies were from the Pilot Production of the Institute of Bioorganic Chemistry National Academy of Sciences of Belarus. HSA was kindly provided by the Republican Applied Research Center of Transfusiology and Medical Biotechnologies (Belarus); rhLF was isolated and purified from milk of transgenic goats carrying the human lactoferrin gene at the Institute of Microbiology, National Academy of Sciences of Belarus.
Salts, bases, acids, and organic solvents were from Belarussian and Russian manufacturers and were at least of the “analytically pure” grade. Buffer solutions were prepared using deionized water with a resistivity of 17–18 MΩ cm, which was obtained using an Arium pro VF ultrapure water system (Sartorius, Germany).
HRP-conjugated aminopenicillins were purified on a Superose 12 column (1.0 × 30.0 cm), which was equilibrated with 0.15-M NaCl, using a fast protein liquid chromatography system (Pharmacia Biotech, Sweden) in an automated mode with a flow rate of 12 mL/h.
ELISA was carried out in 96-well polystyrene strip plates (8-well strips) (Greiner bio-one, Germany) , or Xema Corporate Groupe, Russia).
1H and 13C NMR spectra were recorded in d6‑DMSO using an AVANCE 500 MHz system (Bruker BioSpin, Germany). Chemical shifts were determined relative to solvent residual signals. Mass spectra of the OSu esters were obtained using an Agilent 6410 Triple Quad mass selective detector with an Agilent 1200 SL liquid chromatography system (Agilent Technologies, United States). Molecular weights of proteins and their conjugates were determined by MALDI-TOF mass spectrometry run on a Microflex LRF system (Bruker Daltonik, Germany). Colorimetric signals from microplate wells were measured using AIF-M/340 (Vityaz’, Belarus) and iMark™ (Bio-Rad Laboratories, United States) plate readers.
Bis(2,5-dioxopyrrolidin-1-yl)terephthalate,pPh(OSu)2. A solution of 1.00 g (4.9 mol) of terephthalic acid dichloroanhydride in 30 mL of methylene chloride was chilled in an ice bath and supplemented with 2.00 g (17.4 mmol) of NHS and 2.4 mL (17.4 mmol) of triethylamine. The mixture was stirred in the cold for 30 min and at 20–25°C for 48 h. The pPh(OSu)2 precipitate was collected by filtration, washed with 5 mL of methylene chloride, suspended in 20 mL of methylene chloride, collected by filtration, and dried in a vacuum desiccator with P2O5 at 20–25°C. The yield was 1.37 g (78%) of the diester pPh(OSu)2. Mass spectrum: m/z 383.0 [M + Na]+, 743.1 [2M + Na]+. C16H12N2O8. Calculated: 360.1. 1H-NMR spectrum (d6-DMSO) δ: 2.92 (8H, s, 2 × ‒ОС–СН2–СН2–СО–), 8.34 (4H, s, 4 × CH). 13C‑NMR spectrum (d6-DMSO) δ: 25.6, 130.1, 131.0, 161.0, 170.2.
Bis(2,5-dioxopyrrolidin-1-yl)adipinate, Ad(OSu)2. A solution of 0.44 g (3.0 mmol) of adipic acid in 15 mL of dioxane was supplemented with 0.73 g (6.3 mmol) of NHS, stirred in an ice bath for 10 min, and supplemented with 0.97 mL (6.3 mmol) of diisopropylcarbodiimide. The reaction mixture was stirred at 10–15°C for 1 h and 20–25°C for 20 h. The diisopropyl urea precipitate was collected by filtration, a substantial portion of the solvent was evaporated from the filtrate, and diethyl ether was added. The resulting Ad(OSu)2 precipitate was dissolved in dioxane and precipitated with diethyl ether again. The supernatant was discarded, and the target compound was dried in a vacuum desiccator with P2O5 at 20–25°C. The yield was 0.57 g (56%) of the diester Ad(OSu)2. Mass spectrum: m/z 363.1 [M + Na]+, 703.2 [2M + Na]+. C14H16N2O8. Calculated: 340.1. 1H-NMR spectrum (d6-DMSO) δ: 1.68-1.74 (4Н, m, 2 × –ООС–CH2–СН2–), 2.74 (4Н, t, J 6.1 Hz, 2 × (–ООС–СН2–)), 2.81 (8Н, s, 2 × (–ОС–СН2–СН2–СО–)). 13C-NMR spectrum (d6-DMSO) δ: 23.3, 25.5, 29.7, 168.8, 170.3.
OSu esters of aminopenicillins. Ampicillin trihydrate (50.0 mg, 124 μmol) was added to a solution of 50.0 mg (139 μmol) of pPh(OSu)2 or 50.0 mg (147 μmol) of Ad(OSu)2 in 2 mL of DMF. Then 50 μL (360 μmol) of triethylamine was added, and the reaction mixture was stirred for 3 h. The reaction was monitored by thin-layer chromatography in an ethylacetate–methanol–acetic acid (4 : 2 : 1) system, using UV light and a ninhydrin solution in n-butanol for detection. The solvent was evaporated at 50°C, the pellet was dissolved in methanol, and the product was precipitated with diethyl ether. The supernatant was discarded, and the pellet was washed with diethyl ether and dried in a vacuum desiccator with P2O5 at 20–25°C. The yields were 71 and 90% for Amp-pPh-OSu and Amp-Ad-OSu, respectively.
The Amox derivative was synthesized similarly, using 50.0 mg (119 μmol) of amoxicillin trihydrate and 50.0 mg (139 μmol) of pPh(OSu)2. The yield of Amox-pPh-OSu was 76%.
The isolated OSu esters of aminopenicillins were used to synthesize their conjugates without further purification.
Peroxidase conjugates Amp-pPh-HRP, Amp-Ad-HRP, and Amox-pPh-HRP. A solution (0.5 mL) of 2.0 mg (46 nmol) of HRP in 0.1 M NaHCO3 was supplemented with 25 μL of a solution containing 0.55 mg (920 nmol) of Amp-pPh-OSu, 0.53 mg (920 nmol) of Amp-Ad-OSu, or 0.56 mg (920 nmol) of Amox-pPh-OSu in DMF. The mixture was stirred and incubated at 4°C for 16 h. The conjugates Amp-pPh-HRP, Amp-Ad-HRP, and Amox-pPh-HRP were purified by gel filtration on a Superose 12 column in a fast protein liquid chromatography mode.
Immunogen Amp-pPh-TG. A solution (6.0 mL) of 28.0 mg (42 nmol) of TG in 0.1 M NaHCO3 was supplemented with 16.7 mg (28 μmol) of Amp-pPh-OSu in 0.3 mL of DMF. The mixture was stirred and incubated at 20–25°C for 4 h. The product was desalted on Sephadex G-25 equilibrated with 0.15 M NaCl.
Conjugates Amp-HRP and Amp-rhLF were synthesized according to published protocols [7, 9, 12] with minor modifications. A solution of 2.0 mg (46 nmol) of HRP in 0.1 M MES buffer (pH 6.0) containing 0.15 M NaCl was supplemented with 2.0 mg (5.0 μmol) of ampicillin trihydrate and 2.0 ng (10 μmol) of EDC. The mixture was incubated at 4°C for 16 h, and the product was purified by gel filtration in an automated mode. The immunogen Amp-rhLF was obtained similarly and purified by desalting on a Sephadex G-25 column equilibrated with 0.15-M NaCl.
Conjugate Amp-HSA was synthesized as described previously [19], using 0.8 mg (2.3 μmol) of Amp, 0.7 mg (3.7 μmol) of EDC, 0.8 mg (3.7 μmol) of sNHS, and 15 mg (0.2 μmol) of HSA. The conjugate was purified chromatographically on a Sephadex G-25 column equilibrated with 0.02 M phosphate-buffered saline (PBS).
Buffer solutions. PBS contained 0.05 M of a disodium phosphate–monosodium phosphate mixture (pH 7.4) and 0.15 M NaCl. A blocking buffer contained 5 g/L BSA in PBS. A solution used to immobilize specific antibodies and to perform ELISA contained PBS, 1 g/L BSA, and 0.02% Tween-20. A well washing buffer contained 0.02% Tween-20 in PBS. To obtain a chromogen–substrate mixture, one part of 0.4 mM TMB in DMSO was added to 20 parts of 3.0 mM H2O2 in 0.04 M sodium citrate (pH 4.0). As a stop solution, we used 1 M H2SO4.
Receptor assay. An antimouse Ig sheep antibody solution (5μg/mL in 0.1 M NaHCO3) was added to wells of a polystyrene plate at 100 μL per well. The plate was incubated at 4°C for 16 h, the solution was removed, the wells were washed, the blocking buffer was added at 200 μL per well, and the plate was incubated again at 4°C for 16 h. The solution was removed, 2 μg/mL anti-PBP monoclonal antibody in a buffer was added at 100 μL per well, and the plate was incubated at 4°C for 16 h to immunochemically immobilize the antibody. The solution was removed, and the wells were added with 50 μL of Amp in a concentration range of 0.1–4.0 ng/mL, 25 μL of a HRP conjugate in a selected dilution, and 25 μL of 0.25 μg/mL PBP. The plate was incubated at 20–25°C for 1 h, the wells were washed with the washing buffer, the chromogen–substrate mixture was added at 100 μL per well, the plate was incubated at 20–25°C for 15 min, and the stop solution was added at 100 μL per well. The optical density was measured at 450 nm.
Stability of the β-lactam ring during synthesis of the aminopenicillin derivatives. Aliquots (10 μL) of the reaction mixture were collected during Amp-pPh-OSu synthesis and used to prepare solutions with Amp concentrations of 0.1–4.0 ng/mL. Samples with the same Amp concentrations were prepared using 50 μg/mL Amp solutions in water or 0.1 M NaHCO3, which were preliminarily stored at –20°C for 1 month, 4°C for 16 h, or 37°C for 16 h. The resulting solutions of Amp and its derivatives were tested in the receptor assay as above. The binding activity was calculated using the equation for the cross-reactivity (CR, see “Analytical characteristics of bioanalyses” below), using the Amp exposed to the conditions under study as compound X.
Production of antisera to Amp. Female adult Chinchilla rabbits (body weight 2.5 kg) were kept in breeding facility conditions. Two groups of three rabbits each were immunized with the Amp-pPh-TG or Amp-rhLF conjugate. In the first immunization, 1.0 mg of the immunogen in 1 mL of a 1 : 1 mixture of 0.15 M NaCl and complete Freund’s adjuvant was injected subcutaneously in the neck region. Two to three weeks after, the rabbits were again injected subcutaneously with 0.5 mg of the antigen conjugate in 0.5 mL of a 1 : 1 mixture of 0.15 M NaCl and incomplete Freund’s adjuvant. Subsequent injections of the conjugates in incomplete Freund’s adjuvant were performed at an interval of 4–6 weeks; blood samples were collected on day 7–10 after immunization. Blood was drawn from the marginal ear vein in a tube with clot activators. The serum was separated by centrifugation. The antibody titer was measured by ELISA as the antiserum dilution at which the colorimetric signal A450 was in a range of 1.2–2.0 units.
Indirect ELISA system. The Amp-HSA conjugate was immobilized on the inner surface of a polystyrene plate wells from 100 μL of 0.25-μg/mL Amp-HSA in 0.01 M NaHCO3 by incubating the plate at 4°C for 18 h. The solution was removed, and the blocking buffer was added at 150 μL per well. The plate was incubated at 4°C for 18 h. The wells were added with 50 μL of an Amp solution (0.01–1000 ng/mL) and 50 μL of an antiserum dilution with a titer of 1 : 5 000 to 1 : 100 000. The plate was incubated at 37°C for 1 h. The wells were washed, a peroxidase conjugate of anti-rabbit Ig goat antibodies was added at 100 μL per well, and the plate was incubated at 37°C for 1 h. The wells were washed, added with 100 μL of the chromogen–substrate mixture, incubated at 20–25°C for 15 min, and added with 100 μL of the stop solution. Optical density was measured at 450 nm.
Direct ELISA system. Antirabbit Ig sheep antibodies (5 μg/mL in 0.1 M NaHCO3) were added to wells of a polystyrene plate at 100 μL per well, and the plate was incubated at 4°C for 16 h. The solution was removed, the wells were washed with the washing buffer, the blocking buffer was added at 200 μL per well, and the plate was incubated at 4°C for 16 h. The solution was removed from the wells, 100 μL of a rabbit antiserum to Amp-pPh-ТG (1 : 50 000) or an antiserum to Amp-rhLF (1 : 20 000) were added, and the plate was incubated at 4°C for 16 h. The wells were washed and 50 μL of an Amp solution (0.03–2.0 ng/mL) and 50 μL of an aminopenicillin–HRP conjugate were added. The plate was incubated at 20–25°C for 1 h, the wells were washed, the chromogen–substrate mixture was added at 100 μL per well, the plate was incubated again at 20–25°C for 15 min, and the stop solution was added at 100 μL per well. Optical density was measured at 450 nm.
Analytical characteristics of bioanalyses. The analytical sensitivity was determined as the Amp concentration (ng/mL) at which the binding of an aminopenicillin–HRP conjugate or immobilized Amp-HSA with PBP or specific polyclonal antibodies differed from 100% by three measurement errors B0/(B0 – 3SD). IC50 was obtained as the Amp concentration that inhibited the binding of PBP or anti-Amp antibodies with the Amp or Amox conjugates by 50% when added to wells; i.e., the colorimetric signal was half that from the well that did not contain the antibiotic. CR was calculated using the following equation:
where CR(X) is the cross-reactivity of PBP or specific polyclonal antibodies towards compound X and IC50(Amp) is the Amp concentration (ng/mL) that causes half inhibition of the binding of PBP or specific polyclonal antibodies with the Amp conjugate.
All measurements of the bioanalytical parameters were performed at least in triplicate. The results were processed using the Microsoft Excel spreadsheets. The root-mean-square deviation (SD) is shown with whiskers in Figs. 2 and 4, p < 0.05.
The conjugates and antibodies obtained in this work are available free of charge from the authors.
CONCLUSIONS
New activated esters of carboxy aminopenicillin derivatives were obtained via acylation of Amp and Amox with di-OSu esters of dicarboxylic acids. The derivatives were used to synthesize conjugates with a stable amide bond via a simple procedure. The antibiotic and protein moieties were separated by an aromatic or aliphatic fragment in the conjugates. The β-lactam ring of the antibiotics was shown to remain intact during synthesis of their low-molecular-weight derivatives and subsequent production of their protein conjugates according to the method developed.
The new HRP conjugates Amp-pPh-HRP, Amp-Ad-HRP, and Amox-pPh-HRP, which had an aliphatic or aromatic linker between the antibiotic and protein moieties, were compared with the Amp-HRP conjugate, which was also synthesized in this work via direct (linkerless) attachment of aminopenicillins to amino acid residues of the polypeptide chain. A receptor assay showed that PBP interacted with the linker conjugates better than with the products of direct attachment of aminopenicillins to a carrier protein. Model receptor systems that included Amp-pPh-HRP, Amp-Ad-HRP, or Amox-pPh-HRP ensured a higher penicillin sensitivity as compared with a system that included Amp-HRP.
It is important to note that the receptor systems based on PBP and the aminopenicillin–HRP conjugates are possible to produce as ready-to-use reagent kits and to employ in screening various foods of animal origin for a broad range of β-lactam antibiotics (penicillins and cephalosporins).
Antibodies obtained via long-term immunization of rabbits with the Amp-hrLF conjugate (the carrier protein is 80 kDa) had a low sensitivity in ELISA and extremely high affinity for the Amp-HRP conjugate. To obtain a high-affinity antiserum with a substantial content of highly penicillin-specific antibodies, the linker conjugate Amp-pPh-TG (the carrier protein is 660 kDa) was necessary to use for immunization for the same period of time. Affinity of the resulting polyclonal antibodies for the HRP conjugate that is structurally similar to the immunogen was also somewhat higher than for the other conjugates.
Reliable detection of Amp in a concentration range of 0.03–2.00 ng/mL was achieved in direct ELISA with immunochemically immobilized polyclonal antibodies (to Amp-pPh-TG as an immunogen) and the Amp-Ad-HRP conjugate present in the liquid phase; i.e., the sensitivity was one order of magnitude higher than that of the immunoanalytical systems described in the literature.
Our system may serve as a prototype to develop a reagent kit for ultrasensitive two-step (biospecific binding and colorimetric detection) direct ELISA aimed at detecting penicillins in foods.
REFERENCES
Menkem, Z.E., Ngangom, B.L., Tamunjoh, S.S.A., and Boyom, F.F., Acta Ecol. Sin., 2019, vol. 39, pp. 411–415. https://doi.org/10.1016/j.chnaes.2018.10.004
Decision of the Board of the Eurasian Economic Commission dated February 13, 2018, no. 28. http://pravo.by/document/?guid=3871&p0=F91800044.
Comission Regulation (EU) No. 37/2021, Off. J. Eur. Union, 2010, vol. 5, pp. L15/1-72. https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-5/ reg_2010_37/reg_2010_37_en.pdf.
Riediker, S., Diserens, J.-M., and Stadler, R.H., J. Agric. Food Chem., 2001, vol. 49, pp. 4171–4176. https://doi.org/10.1021/jf010057k
Holstege, D.M., Puschner, B., Whitehead, G., and Galey, F.D., J. Agric. Food Chem., 2002, vol. 50, pp. 406–411. https://doi.org/10.1021/jf010994s
Chiesa, L.M., Nobile, M., Panseri, S., Biolatti, B., Cannizzo, F.T., Pavlovic, R., and Arioli, F., J. Agric. Food Chem., 2016, vol. 64, pp. 2635–2640. https://doi.org/10.1021/acs.jafc.6b00155
Zeng, K., Zhangy, J., Wang, Y., Wang, Z.H., Zhang, S.X., Wu, C.M., and Shen, J.Z., Biomed. Environ. Sci., 2013, vol. 26, pp. 100–109. https://doi.org/10.3967/0895-3988.2013.02.004
Peng, J., Cheng, G., Huang, L., Wang, Y., Hao, H., Peng, D., Liu, Z., and Yuan, Z., Anal. Bioanal. Chem., 2013, vol. 405, pp. 8925–8933. https://doi.org/10.1007/s00216-013-7311-5
Usleber, E., Litz, S., and Martlbauer, E., Food Agric. Immunol., 1998, vol. 10, pp. 317–324. https://doi.org/10.1080/09540109809354995
Strasser, A., Usleber, E., Schneider, E., Dietrich, R., Burk, C., and Martlbauer, E., Food Agric. Immunol., 2003, vol. 15, pp. 135–143. https://doi.org/10.1080/09540100400003493
Bacigalupo, M.A., Meroni, G., Secundo, F., and Lelli, R., Talanta, 2008, vol. 77, pp. 126–130. https://doi.org/10.1016/j.talanta.2008.05.057
Samsonova, Z.V., Shchelokova, O.S., Ivanova, N.L., Rubtsova, M.Y., and Egorov, A.M., Appl. Biochem. Microbiol., 2005, vol. 41, pp. 589–595. https://doi.org/10.1007/s10438-005-0107-4
Cliquet, P., Goddeeris, B.M., Okerman, L., and Cox, E., Food Agric. Immunol., 2007, vol. 18, pp. 237–252. https://doi.org/10.1080/09540100701802908
Cliquet, P., Cox, E., Van Dorpe, C., Schacht, E., and Goddeeris, B.M., J. Agric. Food Chem., 2001, vol. 49, pp. 3349–3355. https://doi.org/10.1021/jf001428k
Dietrich, R., Usleber, E., and Martlbauer, E., Analyst, 1998, vol. 123, pp. 2749–2754. https://doi.org/10.1039/a805166f
Jiao, S.N., Wang, P., Zhao, G.X., Zhang, H.C., Liu, J., and Wang, J.P., J. Environ. Sci. Health B, 2013, vol. 48, pp. 486–494. https://doi.org/10.1080/03601234.2013.761908
Komova, N.S., Berlina, A.N., Zherdev, A.V., and Dzantiev, B.B., Orient. J. Chem., 2020, vol. 36, pp. 21–25. https://doi.org/10.13005/ojc/360103
Li, Y., Xu, X., Liu, L., Kuang, H., Xu, L., and Xu, C., Analyst, 2020, vol. 145, pp. 3257–3265. https://doi.org/10.1039/D0AN00421A
Serchenya, T.S., Gorbachyova, I.V., and Sviridov, O.V., Russ. J. Bioorg. Chem., 2022, vol. 48, pp. 85–95.
Byzova, N.A., Zvereva, E.A., Zherdev, A.V., and Dzantiev, B.B., Appl. Biochem. Microbiol., 2011, vol. 47, pp. 627–634. https://doi.org/10.1134/S0003683811060032
Wang, J., Shen, X., Zhong, P., Zhaodong, Li., Tang, Q., Huang, X., Zherdev, A.V., Dzantiev, B.B., Eremin, S.A., Xiao, Z., Lei, H., and Li, X., Chem. Biol. Technol. Agric., 2021, vol. 8, p. 17. https://doi.org/10.1186/s40538-021-00211-0
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Statement on the Welfare of Animals
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
This work does not contain any studies involving human subjects performed by any of the authors.
Additional information
Translated by T. Tkacheva
Abbreviations: Amp, ampicillin; Amox, amoxicillin; BSA, bovine serum albumin; CR, cross-reactivity; DMF, dimethylformamide; EDC, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide; ELISA, enzyme-linked immunosorbent assay; HRP, horseradish peroxidase; HSA, human serum albumin; rhLF, recombinant human lactoferrin; NHS, N-hydroxysuccinimide; sNHS, N-hydroxysulfosuccinimide; PBP, penicillin-binding protein; PBS, phosphate-buffered saline; TG, thyroglobulin; TMB, 3,3',5,5'-tetramethylbenzidine
Corresponding autors. phone: +375 (17) 395-92-75.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kuprienko, O.S., Serchenya, T.S., Vashkevich, I.I. et al. Conjugates of Aminopenicillins with Proteins: Synthesis, Immunogenic Properties, and Binding to the β-Lactam Receptor and Antibodies. Russ J Bioorg Chem 48, 105–114 (2022). https://doi.org/10.1134/S106816202201006X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S106816202201006X