Abstract
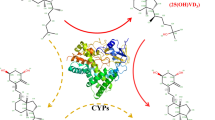
A novel strategy was developed for the preparation of paricalcitol (1α,25-dihydroxy-19-norvitamin D2) via seven-step chemical transformation and one-step microbial transformation. Using vitamin D2 as a starting material, 1α- and 25-hydroxyl groups of paricalcitol were introduced by Pseudonocardia autotrophica CGMCC5098 at the late stage of the synthesis, allowing us to avoid harsh reaction conditions and tedious steps. The overall yield of paricalcitol was 0.75%.
Similar content being viewed by others
References
Fraser, D.R. and Kodicek, E., Nature, 1970, vol. 228, pp. 764–766.
DeLuca, H.F. and Schnoes, H.K., Annu. Rev. Biochem., 1983, vol. 52, pp. 411–439.
Ikekawa, N., Med. Res. Rev., 1987, vol. 7, pp. 333–366.
DeLuca, H.F., Vitamin D, 2nd ed., Feldman D., Pike, J.W., and Glorieux, F.H., Eds., New York: Academic Press, 2005.
Noboru, K., Curr. Bioact. Compd., 2006, vol. 2, pp. 301–315.
Kubodera, N., Molecules, 2009, vol. 14, p. 3869.
Sintov, A. C., Yarmolinsky, L., Dahan, A., and Ben-Shabat, S., Drug Discov. Today, 2014, vol. 19, pp. 1769–1774.
Jones, G., Annu. Rev. Nutr., 2013, vol. 33, pp. 23–44.
Kulesza, U., Sigüeiro, R., Mouriño, A., and Sicinski, R. R., J. Org. Chem., 2013, vol. 78, pp. 1444–1450.
Kabat, M. M. and Radinov, R., Curr. Opin. Drug Discov. Dev., 2001, vol. 4, pp. 808–833.
Goldenberg, M. M., Clin. Ther., 1999, vol. 21, pp. 432–441.
Trillini, M., Cortinovis, M., Ruggenenti, P., Reyes Loaeza, J., Courville, K., Ferrer-Siles, C., Prandini, S., Gaspari, F., Cannata, A., Villa, A., Perna, A., Gotti, E., Caruso, M. R., Martinetti, D., Remuzzi, G., and Perico, N., J. Am. Soc. Nephrol., 2015, vol. 26, pp. 1205–1214.
Bover, J., DaSilva, I., Furlano, M., Lloret, M. J., Diaz-Encarnacion, M.M., Ballarin, J., and Cozzolino, M., Curr. Vasc. Pharmacol., 2014, vol. 12, pp. 313–323.
Toyoda, A., Nagai, H., Yamada, T., Moriguchi, Y., Abe, J., Tsuchida, T., and Nagasawa, K., Tetrahedron, 2009, vol. 65, pp. 10002–10008.
Kubodera, N., J. Syn. Org. Chem. Jpn., 2010, vol. 68, pp. 904–910.
Kubodera, N., Heterocycles, 2010, vol. 80, pp. 83–98.
Li, L., Yue, L., Xue, J., Xie, Z., and Li, Y., Chinese Sci. Bull., 2012, vol. 57, pp. 1616–1619.
Pietraszek, A., Malińska, M., Chodyński, M., Krupa, M., Krajewski, K., Cmoch, P., Woźniak, K., and Kutner, A., Steroids, 2013, vol. 78, pp. 1003–1014.
Deng, Z., People Rep. China Pat., Faming Zhuanli Shenqing, 2014, vol. CN 103896817.
Li, Y., Xue, J., Zhang, X. and Xu, S., Peope Rep. China Pat., Faming Zhuanli Shenqing, 2010, vol. CN 101880253.
Bader, T., Stutz, A., Bichsel, H.-U., and Fu, C., Universitat Zurich Pat., PCT Int. Appl. no. WO 2010009879, 2010.
Chodynski, M., Krupa, M., Krajewski, K., Kubiszewski, M., Kutner, A., Pietraszek, A., and Trzcinska, K., PCT Int. Appl., no. WO 2011016739, 2010.
Kutner, A., Perlman, K. L., Lago, A., Schnoes, H.K., DeLuca, H.F., and Sicinski, R.R., J. Org. Chem., 1988, vol. 53, pp. 3450–3457.
Lu, Q., Feng, H., Wen, P., Sun, B., Wan, Y., and Zhou, A., People Rep. China Pat no. CN 102392053, Faming Zhuanli Shenqing, 2012.
Fujii, Y. and Tamura, T., PCT Int. Appl. no. WO 2007138894, 2007.
Machida, K., Ito, S. F., Yoshikazu, Hirosue, S., and Arisawa, A., PCT Int. Appl. no. WO 2008096695, 2008.
Song, W.-Q., Wang, Y.-H., Liu, D.-N., Fang, W.-Z., and Lu, Q., Russ. J. Gen. Chem., 2016, vol. 86, pp. 173–177.
Wan, Y., Wen, P., Zhang, P., and Lu, Q., Chin. J. Org. Chem., 2012, vol. 32, pp. 1988–1992.
Paaren, H.E., DeLuca, H.F., and Schnoes, H.K., J. Org. Chem., 1980, vol. 45, pp. 3253–3258.
Perlman, K.L., Sicinski R.R., Schnoes, H.K., and DeLuca, H.F., Tetrahedron Lett., 1990, vol. 31, pp. 1823–1824.
Ding, J., Guo, X., Zeng, Z., and Liu, N., Synlett. 2013, vol. 24, pp. 2606–2608.
Author information
Authors and Affiliations
Corresponding authors
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Luo, J.Q., Jiang, F., Song, W.Q. et al. Synthesis of paricalcitol: A novel strategy combining chemical and microbial transformations. Russ J Bioorg Chem 43, 323–327 (2017). https://doi.org/10.1134/S1068162017030104
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162017030104