Abstract
Biological catalysis is an important approach for the production of high-value-added compounds, especially for products with complex structures. Limited by the complex steps of chemical synthesis and low yields, the bioconversion of vitamin D3 (VD3) to calcifediol and calcitriol, which are natural steroid products with high added value and significantly higher biological activity compared to VD3, is probably the most promising strategy for calcifediol and calcitriol production, and can be used as an alternative method for chemical synthesis. The conversion efficiency of VD3 to calcifediol and calcitriol has continued to rise in the past few decades with the help of several different VD3 hydroxylases, mostly cytochrome P450s (CYPs), and newly isolated strains. The production of calcifediol and calcitriol can be systematically increased in different ways. Specific CYPs and steroid C25 dehydrogenase (S25DH), as VD3 hydroxylases, are capable of converting VD3 to calcifediol and calcitriol. Some isolated actinomycetes have also been exploited for fermentative production of calcifediol and calcitriol, although the VD3 hydroxylases of these strains have not been elucidated. With the rapid development of synthetic biology and enzyme engineering, quite a lot of advances in bioproduction of calcifediol and calcitriol has been achieved in recent years. Therefore, here we review the successful strategies of promoting VD3 hydroxylation and provide some perspective on how to further improve the bioconversion of VD3 to calcifediol and calcitriol.
Similar content being viewed by others
Background
Vitamin D3 (VD3) is an essential steroid hormone in the human body and a fat-soluble prohormone. In addition to being obtained from food, VD3 can be synthesized in the human body by ultraviolet irradiation using 7-dehydrocholesterol as a precursor. VD3 has an important role in regulating calcium and phosphorus metabolism and controlling the growth and development of bone cells [1]. However, the biological activity of VD3 is relatively low compared with that of its active forms. In the human body, two-step hydroxylation under the catalysis of cytochrome P450s (CYPs) is required for its full biological activity. VD3 is first converted to 25(OH)VD3 (calcifediol) in the liver, mainly but not exclusively by CYP2R1, and then further converted to 1α,25(OH)2VD3 (calcitriol) by CYP27B1 in the kidney to gain its full activity [2]. VD3 helps treat osteoporosis, chronic renal dysfunction and other diseases [3, 4]. Supplementation with the hydroxylation products of VD3 instead of VD3 itself eliminates the need for the hydroxylation process in the human body, making it important for patients with abnormalities in vitamin D metabolism. It has been reported that supplementation with calcitriol is helpful for the treatment of liver [5] and kidney disorders [6], and calcifediol is used for the treatment of calcium disorders, such as rickets [7]. In addition, calcifediol or calcitriol supplementation is able to prevent cartilage diseases, improve egg production and egg quality of laying hen, and enhance reproductive performance of breeding swine in the livestock and poultry production [8,9,10].
At present, calcifediol and calcitriol are mainly produced by chemical synthesis, which requires complex protection and deprotection steps for the regioselective introduction of hydroxyl groups, especially for hydroxylation on C-1, limiting the industrial production [11, 12]. For example, starting with hydrindenylpropanol, Lythgoe et al. [13] synthesized des-AB-cholestane-8β,25-diol, which could be further used as a substrate for the synthesis of calcifediol. The synthesis process of des-AB-cholestane-8β,25-diol requires multiple steps and further conversion to calcifediol. Calcitriol can be obtained by condensation of 1α-hydroxylated phosphine oxide with 25-hydroxy ketone [14]. However, the substrates also require multiple steps for synthesis. Thus, compared with chemical synthesis, the bioconversion of VD3 is a successive process that comprises two (25- and 1α) or one (only 25-) hydroxylation steps. In addition, the yield of chemical synthesis of calcifediol is low (the classical approach by photochemical ring opening of steroidal Δ5,7-dienes yields less than 1%) [15]. Therefore, increasing attention is being paid to the bioconversion of VD3 to calcifediol and calcitriol. Though hydroxylated sterols could be synthesized by transgenic Arabidopsis plants with non-heme monooxygenase [16], VD3 has not yet been reported as a substrate. Microorganisms have been a powerful tool for VD3 hydroxylation research, and in some work, the VD3 conversion rate was remarkable. Therefore, a bioconversion process with VD3 as the substrate and microorganisms as the chassis cells is promising for its simplicity and low pollution.
In the past few decades, a large number of strains expressing VD3 hydroxylases have been developed for VD3 bioconversion. A common strategy is to screen organisms with VD3 hydroxylation capabilities and then examine their calcifediol and calcitriol production abilities by fermentation. Furthermore, the characterization of VD3 hydroxylases from different sources and their heterologous expression in different host cells are also topics of particular interest in this field. These VD3 hydroxylases mainly refer to cytochrome P450s. In addition, steroid C25 dehydrogenase (S25DH) was also reported to have the ability to hydroxylate VD3 at the C-25 position. However, S25DH is sensitive to oxygen, causing a high cost for calcifediol production [17]. CYPs are the primary enzymes currently used for bioconversion of VD3 to its active forms. A variety of CYPs from mammals and bacteria (mainly from actinomycetes) that are capable of converting VD3 to calcifediol and calcitriol have been identified, and highly active mutants have been constructed. Enhanced calcifediol and calcitriol production could further benefit from the rapid development of creating new enzymes and new biochemical reactions via computational redesign of enzymes.
In general, enzymes and whole cells are two categories of biocatalysts commonly used for bioconversion [18, 19]. Here, we review advances in the characterization and engineering of VD3 hydroxylase that plays a key role in VD3 bioconversion, and progress in whole-cell bioconversion, which can use inexpensive and abundant raw materials and avoiding the addition of expensive cofactors.
VD3 hydroxylases and their protein engineering
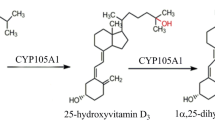
For the synthesis of complex compounds, including calcifediol and calcitriol, enzymatic conversion has high regio- and stereoselectivity when compared with chemical synthesis. VD3 hydroxylases, most of which are CYPs, play a central role in the bioconversion of VD3 into calcifediol and calcitriol. Cytochrome P450s (CYPs) are a class of heme-containing enzymes with similar structures. CYPs catalyze a variety of reactions, mostly monooxygenation reactions, and have a wide range of substrate specificities [20]. The main CYPs related to VD3 hydroxylation are shown in Table 1 and Fig. 1.
VD3 bioconversion process. VD3 is sequentially converted to calcifediol and calcitriol by CYPs. Some uncommon CYPs can also catalyze the conversion of VD3 to calcitrol with 1α(OH)VD3 as the intermediate. Many CYPs, such as CYP27B1, can catalyze hydroxylation at two sites, as they have relatively wide substrate specificity and regioselectivity
Mammalian and bacterial CYPs have been widely utilized for the bioproduction of calcifediol and calcitriol, the active forms of VD3. Mammalian CYPs have attracted great attention as they are associated with VD3 metabolism related diseases. Bacterial CYPs are also of great importance because of their enormous functional diversity. A unique property of bacterial CYPs is that carbon monoxide (CO), SKF-525-A and metyrapone inhibit the hydroxylase activities of bacterial CYPs. Therefore, reduced CO difference spectroscopy can be used to identify bacterial CYPs that function as VD3 hydroxylases [21, 22]. To simplify the purification of CYPs, especially mammalian CYPs, the heterologous expression of CYPs in model organisms can increase protein yield and reduce the cost [23]. Escherichia coli and yeast are ideal host cells for heterologous expression of CYPs, as they grow quickly and genetic tools for their modifications have been well developed. Given that the hydroxylation reaction of CYPs requires redox proteins to deliver electrons, it is of great importance to improve VD3 conversion rates using appropriate redox partners and coenzyme regeneration systems. Because of the existence of abundant redox proteins, actinomycetes are also becoming popular for heterologous expression of VD3 hydroxylases. With an increased understanding of the relationship between the protein structures of CYPs and their hydroxylation abilities, protein engineering has been widely applied to the evolution of CYPs in the laboratory, which aims to improve the activity of CYPs for enhanced VD3 bioconversion.
CYPs from mammals
Mammalian VD3 hydroxylases such as CYP27A1, CYP27B1, CYP2D25, CYP2J3 and CYP2R1 have been found to be able to catalyze C-25 or C-1α hydroxylation [24,25,26,27,28]. The CYP27A1 was the first mammalian CYP to be thoroughly studied. Saccharomyces cerevisiae expressing a modified rat CYP27A1 localized in microsomes could convert 1α(OH)VD3 to calcitriol when adrenodoxin (ADX) and NADPH-adrenodoxin reductase (ADR) were coexpresseded [24]. The ADRs from yeast and rats were unable to transfer electrons for rat CYP27A1. However, the purified bovine ADR and ADX facilitated the reaction. Therefore, the mitochondria-targeting signals of bovine ADR and ADX were removed to construct an electron transfer chain between the cytoplasm and the modified CYP27A1 that was incorporated into microsomes. The modified rat CYP27A1 could convert 1α(OH)VD3 to calcitriol with the help of the newly constructed electron transfer chain. In one study, human CYP27A1, which also belongs to the CYP27 family was expressed in E. coli to examine its enzyme activity with VD3, and only a low activity of human CYP27A1 toward VD3 (with Vmax of 0.27 mol/min/mol P450) was observed in this reaction system [29]. Although the 25-hydroxylation activity of human CYP27A1 was notably lower than that of several other mammalian CYPs such as human CYP2R1 (kcat = 0.61 min−1 for 25-hydroxylation activity toward vitamin D3 with CYP27A1 compared to kcat = 0.27 min−1 with CYP2R1), human CYP27A1 could perform 1α-hydroxylation toward calcifediol. Besides calcifediol and calcitriol, other products of human CYP27A1 hydroxylation include 26(OH)VD3, 24R,25(OH)2VD3, 27(OH)VD3, 25,26(OH)2VD3, 25,27(OH)2VD3, 27-oxo-VD3 and a dehydrogenated form of VD3, making the recovery of calcifediol or calcitriol much more difficult. In addition, ergosterol in the yeast membrane might inhibit the VD3 bioconversion as a potential substrate for CYP27A1. Therefore, compared with yeast, E. coli may be a better host for heterologous expression of mammalian CYPs due to its lack of the P450 genes and steroids [30].
Mitochondrial CYP27A1 in human liver is considered to be one of the CYPs that performs C-25 hydroxylation, while CYP27B1, another member of the CYP27 family, is responsible for hydroxylation at the C-1α position. However, Uchida et al. found that, by adding purified ADR and ADX to the reaction system, mouse CYP27B1 could perform both 25-hydroxylation and 1α-hydroxylation, similar to CYP27A1, suggesting that 1α-hydroxylation and 25-hydroxylation of vitamin D3 are closely related [23]. This observation led to the hypothesis that vitamin D3 can be arranged in the opposite direction of the substrate-binding pocket of CYP27B1. In addition, molecular chaperone GroEL/ES greatly enhanced the expression of mouse CYP27B1 in E. coli, making great progress for heterologous expression of CYPs in the future. In addition to mouse CYP27B1, human CYP27B1 also has 1α-hydroxylation activity [25]. However, the variant human CYP27B1 with CYP27B1T409I or CYP27B1Q65H amino acid substitution caused vitamin D 1α-hydroxylase deficiency (Vitamin D-dependent Rickets Type 1). The identification of amino acids that interact with the substrate could provide structural clues for the protein engineering of CYPs. The Ser408 residue in mouse CYP27B1 and the Thr409 residue in human CYP27B1 were found to be responsible for the C-1α hydroxylation by forming a hydrogen bond with the 25-hydroxyl group of calcifediol [31]. However, the problem with the low expression of human CYP27B1 still exists. Different strategies were thus employed to optimize the expression of human CYP27B1. High expression of human CYP27B1, shown by conversion to calcitriol with a yield of above 6000 pmol/mg protein in 30 min, was finally achieved by coexpressing GroEL/ES and human ADX/ADR rather than bovine ADX/ADR [32]. The addition of cardiolipin to the reconstituted system markedly lowered (threefold) the Km of human CYP27B1 for calcifediol, suggesting that the activity of CYPs could also be influenced by phospholipid composition.
Several other mammalian CYPs were also found to be relevant to VD3 hydroxylation. Both human CYP2R1 and human CYP27A1 can catalyze the 25-hydroxylation of VD3. However, the 25-hydroxylation activity of CYP2R1 on VD3 is five times higher than that of CYP27A1. In addition, a mutation in the human CYP2R1 gene results in vitamin D-dependent rickets [33]. These data indicate that CYP2R1 also plays an important role in calcifediol biosynthesis in human body [28]. The GroEL/ES chaperonin can also be used to increase the expression level of human CYP2R1 in E. coli [34]. To understand which amino acid residues are responsible for the substrate specificity of CYP2R1, the crystal structure of CYP2R1 in complex with VD3 was solved. VD3 binds in an elongated conformation with the aliphatic side-chain pointing to the heme. The substrate access channel is covered by the ordered B′-helix. The conserved active site in a closed conformation of CYP2R1 is mainly composed of hydrophobic amino acid residues. Porcine CYP2D25 was expressed in yeast by Hosseinpour [26] and Araya [35], and the substitution of five residues in substrate binding site 3 (SRS-3) inhibited its 25-hydroxylase activity. Besides 25-hydroxylase towards VD3, CYP2D25 is also able to convert calcifediol into calcitriol, 25,26(OH)2VD3, and 25,27(OH)2VD3.
Just like CYP2R1 as VD3 25-hydroxylase, the recombinant rat CYP2J3 shows a 25-hydroxylation activity when it was coexpressed with GroEL/ES in E. coli [27]. The turnover numbers were 3.3 and 22 toward VD3 and 1α(OH)VD3, respectively. Human CYP2J2 shows 73% amino acid homology with CYP2J3. After the deletion of a putative membrane anchor region in the N-terminus and the coexpression of GroEL/ES, efficient expression of CYP2J2 was achieved. However, human CYP2J2 does not seem to be a principal VD3 25-hydroxylase [36]. The CYP2C11, a male-specific hepatic recombinant microsomal vitamin D 25-hydroxylase, has a substrate preference toward VD3 over 1α(OH)VD3 [37]. The 25-hydroxylation activity of the CYP2C11 towards VD2 was higher than that towards VD3 (25-hydroxylation rate of 0.49 ± 0.03 nmol·nmol−1·1.5 h−1 for VD2 compared with 0.07 ± 0.02 nmol·nmol−1·1.5 h−1 for VD3). The examples of CYP2J2 and CYP2C11 imply that some CYPs from mammals take VD2 as a prioritized substrate and thus might not be the best choice for the hydroxylation of VD3.
CYPs from microorganisms
CYPs existing in a variety of organisms are involved in many biosynthetic pathways [20, 25]. Since the initial discovery of the VD3 hydroxylation in Amycolata sp. and Streptomyces sp. strains, CYPs from actinomycetes have been widely used for the VD3 bioconversion process [21, 22, 38, 39]. The 25-hydroxylation activity of VD3 by CYP105A2 (with the highest yield of 10%) was observed by heterologous expression of CYP105A2 from Amycolata autotrophica (Pseudonocardia autotrophica) in Streptomyces lividans [40]. Cytochrome P450SU-1 (CYP105A1) from Streptomyces griseolus was found to convert VD3 to 25(OH)VD3 when expressed in E. coli [41]. Although the amino acid sequences of CYP105A1 and CYP105A2 share 55% identity, there is a clear difference in catalytic activity between CYP105A1 and CYP105A2. Besides the 25-hydroxylation of VD3, CYP105A1 also shows 1α-hydroxylation of calcifediol. In addition, CYP105A1 can also catalyze the hydroxylation of VD2 with a relatively high Vmax/Km value (0.142 L−1·min−1/mol P450, compared to 30 L−1·min−1/mol P450 for VD3). The CYP107CB2 from Bacillus lehensis G1 was classified into the CYP107 subfamily and showed 25-hydroxylation activity of both VD3 and 1α(OH)VD3 [42].
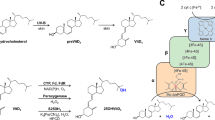
CYPs that catalyze the hydroxylation of VD3 share a common catalytic mechanism with most CYPs. The catalytic cycle occurs on the heme prosthetic group which is linked by a conserved cysteine [43], but the catalytic specificity of CYPs mainly depends on their substrate binding pockets [44] (Fig. 2). Based on the catalytic mechanism, the catalytic specificity of CYP for VD3 can be improved by directed evolution of CYPs.
Structural analysis of CYP105A1 in complex with calcitriol found that Arg73 and Arg84 along with Arg193 were located in the distal pocket of CYP105A1. The Arg73 and Arg84 may have an inhibitory effect on activity, while Arg193 is required for the activity [39, 45, 46]. Therefore, a series of CYP105A1 variants focusing on residues Arg73 and Arg84 were made to achieve a higher activity (with relative kcat/Km compared with wild type ranging from 1 min−1·μM−1 to 43 min−1·μM−1 with 1α(OH)VD3 and calcifediol as substrate). Compared with the wild-type CYP105A1, the activity of CYP105A1R84A variant greatly increased, with activity of 25-hydroxylation toward 1α(OH)VD3 and 25-hydroxylation toward calcifediol are approximate 27-fold and 16-fold than CYP105A1WT, respectively. The improved activity can be attributed to the loss of two hydrogen bonds, which resulted in a new transient binding site for both the substrate and product [45]. The variant of CYP105A1 containing amino acid substitutions R73V and R84A exhibited much higher hydroxylation activities at positions C-1α and C-25 compared to the wild type; the CYP105A1R84A variant also shows a kcat/Km value for 25-hydroxylation that is 435-fold higher than that of the wild type; the CYP105A1R84A mutant also shows a higher 1α-hydroxylation capability than any single mutant. The amino acid residue at position 73 affects the location and conformation of the substrate in the reaction center, while the amino acid residue at position 84 affects the location and conformation of the transient binding site. In addition, the CYP105A1R73VR84A [39] also catalyzes the hydroxylation at position C-26, with 1α,25(R),26(OH)3VD3 and 1α,25(S),26(OH)3VD3 as products. The presence of C-26 hydroxylation activity could be caused by the rotation of a secosteroid skeleton and an evident conformational change of the side chain in the heme pocket.
Inspired by the engineering of CYP105A1R73VR84A, arginine residues around the substrate entrance and active site of CYP105D7 were mutated to generate a double mutant CYP105D7R70AR190A [47]. The CYP105D7R70AR190A achieved an almost ninefold increase in the conversion rate of testosterone, suggesting that the arginine residues around the substrate entrance and active site play important roles in determining the functions of the CYPs. Interestingly, the CYP105A1 is not the only VD3 hydroxylase found in P. autotrophica. Vdh (CYP107BR1) from P. autotrophica NBRC 12743 also has the capability of converting VD3 into calcifediol and calcitriol [48]. Different from other VD3 hydroxylases, the Vdh has no preferred regio-specific hydroxylation of VD3.
A Vdh variant designated as Vdh-K1 with four amino acid substitutions was obtained by means of random mutagenesis, with its activity an order of magnitude higher than that of wild-type Vdh. Yasutake et al. then analyzed the structures of Vdh and Vdh-K1 [49]. The wild-type Vdh showed an open conformation, and the distal heme pocket was exposed to solvent with or without substrate. In contrast, Vdh-K1 shows a closed conformation, increasing the substrate binding affinity and catalytic activity. In addition, the combination of VD3 and calcifediol with Vdh-K1 is in an antiparallel orientation. It is worth noting that the four substituted residues of Vdh-K1 are dispersed throughout the protein but result in a closed conformation of the heme pocket. The VdhT107A variant, which was made by modifying the putative ferredoxin-binding site of Vdh, also exhibited a closed conformation similar to Vdh-K1 bound to VD3 [50]. The ferredoxin-binding surfaces of VdhT107A seem to show a stronger positive potential that is conducive to the binding of ferredoxin when compared with the structures of Vdh-K1. Although the enzyme activity of Vdh-K1 was much higher than that of wild-type Vdh, its expression level in Rhodococcus erythropolis cells was low, in contrast with that of VdhT107A, which was approximately 70% of VdhWT. In addition, VdhT107A has an improved activity that is comparable to that of Vdh-K1, and achieves a similar expression that is comparable to Vdh simultaneously.
The above examples are the modification of the substrate binding pocket. Nevertheless, the engineering technology of CYPs has been greatly developed. Drawing on these technologies, it may be possible to use allosteric regulation, decoy-based small molecules etc., for reference in the hydroxylation of VD3 in the future. For example, the conformational transition of CYP3A4 is induced by the allosteric ligand progesterone (PGS), and CYP3A4-PGS conjugates thus acquire a twofold higher capacity to oxidize testosterone than CYP3A4 [51]. Inert decoy molecules that have a native substrate-like structure, can constrain substrates, such as benzene and benzene derivatives, into the suitable positions to facilitate the catalysis by targeting the enzyme substrate pocket to induce an active enzyme intermediate [52, 53]. If the above technologies are combined into the catalytic reaction of VD3, it may be possible to expand the types of CYPs that can be used to catalyze VD3, inspiring future research that may have unforeseen benefits.
Conversion of VD3 by steroid C25 dehydrogenase
Rugor et al. reported that steroid C25 dehydrogenase (S25DH) from the β-proteobacteria Sterolibacterium denitrificans was able to catalyze the regioselective hydroxylation of sterols and their derivatives [17]. The reaction was carried out in a batch reactor supplemented with purified S25DH (as the enzyme), VD3 (substrate), hydroxypropyl-β-cyclodextrin (solubilizer), 2-methoxyethanol (organic cosolvent) and K3[Fe(CN)6] (electron acceptor). A calcifediol titer of 1.4 g/L was achieved after a 162-h incubation, with a yield as high as 99%.
Compared with CYPs, S25DH has advantages as well as disadvantages. The catalytic activity of S25DH is higher than that of the CYPs reported thus far. In addition, the regioselectivity toward the C-25 position of VD3 is higher in S25DH than that in CYPs, making the recovery of calcifediol much easier. However, S25DH is sensitive to oxygen, so the bioconversion process must be carried out under anaerobic conditions [54]. Jacoby et al. developed a platform for the overproduction of four steroid C-25 hydroxylases (S25DH1 and three isoenzymes S25DH2, S25DH3, and S25DH4) by coexpressing an essential chaperone in the betaproteobacterium Thauera aromatica K172 [55]. The crude extract from T. aromatica that overexpresseed S25DH1 achieved a reaction rate that was 6.5-fold higher than that of the wild-type bacterial extract. However, the oxygen intolerance and high cost prevent S25DH from being applied for the industrial production of calcifediol.
Whole cell conversion of VD3 into calcifediol and calcitriol
A whole-cell conversion system expressing the CYPs of interest has advantages over enzymatic conversion. It avoids adding the expensive cofactor NAD(P)H, and the hydrogen dioxide produced by the uncoupling reactions does not accumulat because of the presence of catalase in the cell. Therefore, increasing attention has been given to the whole cell conversion of VD3 into calcifediol and calcitriol. initially, attempts were made to screen natural microbial strains that are able to convert VD3 to calcifediol and calcitriol in nature. With the development of genome editing and synthetic biology, the production of calcifediol and calcitriol has been further improved by either overexpressing heterogenous VD3 hydroxylase genes or altering the electron transfer chains.
Conversion of VD3 to calcifediol and calcitriol by microbes isolated in nature
Sasaki et al. reported the bioconversion of VD3 derivatives for the first time [21]. After screening approximately 300 Streptomyces strains, S. sclerotialus FERM BP-1370 and S. roseosporus FERM BP-1574 were found to be able to introduce hydroxy group at C-1α position of calcifediol and C-25 position of 1α(OH)VD3, respectively. Although VD3 derivatives were used as substrates rather than VD3 itself, this work demonstrated that microorganisms could be used for the production of hydroxylated derivatives of VD3. By further screening within bacterial and fungal strains, P. autotrophica FERM BP-1573 was chosen from twelve VD3-hydroxylating strains and achieved a calcifediol titer of 8.3 mg/L and a calcitriol titer of 0.17 mg/L in a 200 L fermenter [22].
With the ever-increasing number of newly discovered VD3-hydroxylating strains, the production of calcifediol and calcitriol has been further improved. The actinomycete Kutzneria albida produced 70.4 mg/L calcifediol and 2.0 mg/L calcitriol in an optimized conversion process [56]. When using Pseudonocardia sp. KCTC 1029BP as a whole-cell catalyst, calcifediol production further rose to 356 mg/L, and calcitriol production rose to 61.87 mg/L, with bioconversion yields of 59.4% and 30.94%, respectively [57, 58]. More recently, Tang et al. obtained up to 830 mg/L calcifediol using Bacillus cereus zju 4-2, representing a significant increase in calcifediol production [59] (Table 2). In addition to calcifediol and calcitriol, some side products of VD3 hydroxylation such as 2α,25(OH)2VD3 could also be synthesized, increasing the difficulty of product recovery [60]. It is of great significance to reduce the formation of side products by isolating a microbial strain with high regioselectivity.
Though many attempts have been made to enhance the calcifediol and calcitriol production, the acquirable concentrations of calcifediol and calcitriol in the fermentation broth are not yet satisfactory. Therefore, a series of fermentation parameters have been optimized to further improve the production of calcifediol and calcitriol. The influence of bioconversion buffers, solubilizers, and metal salts on calcitriol production has also been examined, and optimized bioconversion medium was finally been obtained. The optimization of three key variables (aeration rate, resting cell concentration and temperature) in Pseudonocardia fermentation improves the VD3 bioconversion [57, 58].
Conversion of VD3 to calcifediol and calcitriol by engineered strains
With the development of genomics and synthetic biology, engineering of microbial cells provides a new strategy to improve the production of calcifediol and calcitriol. Besides previously mentioned heterologous expression of CYPs for purpose of enzymatic activity characterization and structural analysis, design of engineered strains for whole-cell bioconversion could be applied for industrial-scale production of calcifediol and calcitriol. Actinomycete chassis cells such as S. lividans and R. erythropolis have stable redox environments, which may explain why most bacterial VD3-hydroxylating CYPs have been discovered in actinomycetes; thus, these cells could be promising chassis cells for calcifediol and calcitriol production. When expressing CYP105A1R73VR84A in S. lividans, approximately 7.7 mg/L calcifediol was observed in the culture [39]. R. erythropolis is another industrially important actinomycete that exhibits resistance to organic solvents, so it was used as a host cell to convert VD3 to calcifediol and calcitriol [61] (Fig. 3). The permeability of VD3 into the cytoplasm is a rate-limiting step for VD3 bioconversion [62]. In addition, the electron carrier ferredoxin and the NADH-regeneration system also play essential roles in the conversion of VD3 to its bioactive form. When Vdh was coexpressed with the redox partners AciBC and glucose dehydrogenase GlcDH-IV in nisin-treated R. erythropolis cells, 1176.5 μg calcifediol was obtained, representing an improved calcifediol production when compared with the wild-type strain [61]. Deletion of CYP-sb3 gene dramatically impaired the VD3 bioconversion of by Sebekia benihana, suggesting that CYP-sb3a functions as a VD3 hydroxylase. When CYP-sb3a was expressed in Streptomyces coelicolor that originally had no VD3 hydroxylation ability, calcifediol and calcitriol were detected in the recombinant S. coelicolor cells [63]. B. megaterium is another popular host that has been used for VD3 bioconversion (Fig. 4). When B. megaterium expressing human CYP27A1 was used as whole-cell catalyst, 80.81 mg/L VD3 was converted to its bioactive form. In addition, B. megaterium overexpressing CYP109A2T103A produced 282.7 mg/L calcifediol in 48 h under optimized conditions. Though there are still technical challenges for calcifediol and calcitriol production by engineered strain, designing better artificial strains is a promising strategy to further enhance the calcifediol and calcitriol production by designing better artificial strains.
NAD(P)H-regeneration system enhances the bioconversion of VD3 to calcifediol and calcitriol in engineered R. erythropolis. Sufficient reducing equivalents (NADH/NADPH) obtained by the NAD(P)H-regeneration system (GlcDH from B. megaterium) provide more electrons required for the catalysis of the hydroxylation reaction. Moreover, nisin treatment contributes to increased permeability of the cell membrane
B. megaterium is used as a host cell for VD3 bioconversion. The electrons required for CYP activity are supplied by the endogenous cytochrome P450 reductase (CPR): NADPH-dependent diflavin reductase [76]. Quillaja saponin is used as a membrane-solubilizing agent
With the help of the NADPH-regenerating system, approximately 40% VD3 was converted to calcifediol in 3 hours. The CYP109A2 [64] and CYP109E1 [65] from Bacillus megaterium also exhibited high VD3 bioconversion abilities. After 24 h of whole-cell catalysis, the conversion rates of CYP109A2 and CYP109E1 reached 76% and 95%, respectively. However, NMR analysis showed that CYP109E1 catalyzed the hydroxylation of VD3 at both C-24 and C-25. Approximately twofold more calcifediol was produced in the CYP109E1I85A variant obtained by site-directed mutation than in the wild-type CYP109E1 through whole-cell bioconversion, suggesting that the CYP109E1I85A variant achieves improved selectivity for 25-hydroxylation of VD3. In addition, after a 48-h incubation under optimized conditions, 282.7 mg/L calcifediol was produced by CYP109A2T103A, a more efficient variant obtained by protein engineering of CYP109A2 [66].
The highest yield by engineered strains was achieved by the protein engineering of Vdh (CYP107BR1). For the Vdh and its variants, when R. erythropolis cells expressing VdhT107A were treated with nisin for VD3 conversion, 573 mg/L calcifediol was produced in 2 hours, representing a significantly improved bioconversion rate. Compared with wild-type Vdh, a 1.7-fold of calcifediol production was observed in shorter time (2 h compared with 16 h).
Whether by isolated strains or engineered strains, the efficiency of the VD3 bioconversion process is limited in different levels, mainly genes expression, enzyme activity, efficiency of CYP-redox partner interactions, the NAD(P)H regeneration system and the substrate transportation. Since various efforts have been made in optimizations, a combination of different methods could result in a higher yield. For example, with E. coli as the host, not only by expression of high enzymatic activity CYPs which are assisted with adequate Fdr and Fdx, the introduction of GroEL/ES system along with the mutation of genes include acrAB and tolC [67], whose expression products compose a efflux pump system which play positive roles simultaneously. The deletion of acrAB and tolC in E. coli BL21star(DE3) works similar to the nisin treatment for R. erythropolis, which can increase in the intracellular substrate concentration. In addition, in the fermentation process, the addition of Fe2+ and 5-aminolevulinic acid (5-ALA) [68], which is precursor of heme can be adopted in most cases to ensure the functional expression of CYPs.
In addition to improving the transportation of substrates, there are other potential methods of improving VD3 bioconversion. CYPs displayed on the cell surface of E. coli [69, 70] will provide alternative solution for the transportation of VD3. In the meanwhile, this method could be also used for the screening of highly efficient VD3 hydroxylases. The fusion of CYPs and redox partners could construct a self-sufficient P450 [71], which is promising for future methods of VD3 bioconversion.
Conclusions
Calcifediol and calcitriol have bright market prospects in the poultry farming and pharmaceutical industries. Compared with the traditional chemical synthesis, bioconversion of VD3 to calcifediol and calcitriol represents a promising and eco-friendly technology, and will contribute to the sustainable development goals of United Nations. Given that VD3 hydroxylase plays a key role in the bioconversion of VD3 to calcifediol and calcitriol, a series of CYPs have been modified by protein engineering to increase their catalysis of VD3 hydroxylation. In addition, the VD3 permeability and the electron transfer from NADH to VD3 hydroxylase are also of great importance in determining the rate of VD3 bioconversion. Therefore, the lipid II-targeting antibiotic nisin, redox partners and NAD(P)H regeneration systems are employed to further improve the VD3 bioconversion by enhancing the VD3 uptake and optimizing the electron transfer pathway. High-throughput screening technology has shown its advantages in the field of strain screening [72], and high-throughput screening based on the calcifediol/calcitriol sensor can be used to screen microbial strains that are capable of converting VD3 to calcifediol or calcitriol more efficiently.
With increasing pressure to conserve energy and protect the environment, increasing attention is being paid to the bioproduction of value-added chemicals in a more eco-friendly manner [73, 74]. The “next generation industrial biotechnology” based on moderately halophilic bacteria, which can avoid sterilization and the use of fresh water, exhibits advantages over the current industrial biotechnology. Given that a large number of VD3-hydroxylating strains belong to actinomycetes, efficient screening of halophilic actinomycetes could be a promising strategy to obtain more ideal calcifediol/calcitriol-producing strains. In addition, engineering of the well-studied halophilic bacteria such as Halomonas bluephagenesis could be another promising strategy to further improve the calcifediol/calcitriol production in the future.
Availability of data and materials
Not applicable.
Abbreviations
- VD3 :
-
Vitamin D3
- CYPs:
-
Cytochrome P450s
- S25DH:
-
Steroid C25 dehydrogenase
References
Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96(4):507–15.
Zhu JG, Ochalek JT, Kaufmann M, Jones G, DeLuca HF. CYP2R1 is a major, but not exclusive, contributor to 25-hydroxyvitamin D production in vivo. Proc Natl Acad Sci U S A. 2013;110(39):15650–5.
Hsu C-Y, Chen L-R, Chen K-H. Osteoporosis in patients with chronic kidney diseases: a systemic review. Int J Mol Sci. 2020;21(18):6846.
Hou Y-C, Wu C-C, Liao M-T, Shyu J-F, Hung C-F, Yen T-H, Lu C-L, Lu K-C. Role of nutritional vitamin D in osteoporosis treatment. Clin Chim Acta. 2018;484:179–91.
Guo J, Ma Z, Ma Q, Wu Z, Fan P, Zhou X, Chen L, Zhou S, Goltzman D, Miao D. 1, 25 (OH) 2D3 inhibits hepatocellular carcinoma development through reducing secretion of inflammatory cytokines from immunocytes. Curr Med Chem. 2013;20(33):4131–41.
Christiansen C, Rødbro P, Christensen M, Hartnack B, Transbøl I. Deterioration of renal function during treatment of chronic renal failure with 1, 25-dihydroxycholecalciferol. The Lancet. 1978;312(8092):700–3.
Brandi ML, Minisola S. Calcidiol [25 (OH) D3]: from diagnostic marker to therapeutical agent. Curr Med Res Opin. 2013;29(11):1565–72.
Coffey J, Hines E, Starkey J, Starkey C, Chung T. Feeding 25-hydroxycholecalciferol improves gilt reproductive performance and fetal vitamin D status. J Anim Sci. 2012;90(11):3783–8.
Sugiyama T, Kusuhara S, Chung TK, Yonekura H, Azem E, Hayakawa T. Effects of 25-hydroxy-cholecalciferol on the development of osteochondrosis in swine. Anim Sci J. 2013;84(4):341–9.
Chen C, Turner B, Applegate T, Litta G, Kim W. Role of long-term supplementation of 25-hydroxyvitamin D3 on egg production and egg quality of laying hen. Poult Sci. 2020;99(12):6899–906.
Bouillon R, Okamura WH, Norman AW. Structure-function relationships in the vitamin D endocrine system. Endocr Rev. 1995;16(2):200–57.
Kametani T, Furuyama H. Synthesis of vitamin D3 and related compounds. Med Res Rev. 1987;7(2):147–71.
Lythgoe B, Roberts DA, Waterhouse I. Calciferol and its relatives. Part 20. A synthesis of Windaus and Grundmann’s C 19 ketone. J Chem Soc Perkin Trans. 1977;1(23):2608–12.
Baggiolini EG, Iacobelli JA, Hennessy BM, Uskokovic MR. Stereoselective total synthesis of 1. Alpha., 25-dihydroxycholecalciferol. J Am Chem Soc. 1982;104(10):2945–8.
Zhu G-D, Okamura WH. Synthesis of vitamin D (calciferol). Chem Rev. 1995;95(6):1877–952.
Beste L, Nahar N, Dalman K, Fujioka S, Jonsson L, Dutta PC, Sitbon F. Synthesis of hydroxylated sterols in transgenic Arabidopsis plants alters growth and steroid metabolism. Plant Physiol. 2011;157(1):426–40.
Rugor A, Tataruch M, Staroń J, Dudzik A, Niedzialkowska E, Nowak P, Hogendorf A, Michalik-Zym A, Napruszewska D, Jarzębski A. Regioselective hydroxylation of cholecalciferol, cholesterol and other sterol derivatives by steroid C25 dehydrogenase. Appl Microbiol Biotechnol. 2017;101(3):1163–74.
Lin B, Tao Y. Whole-cell biocatalysts by design. Microb Cell Fact. 2017;16(1):1–12.
Gao C, Zheng T. Drug metabolite synthesis by immobilized human FMO3 and whole cell catalysts. Microb Cell Fact. 2019;18(1):1–12.
Di Nardo G, Gilardi G. Natural compounds as pharmaceuticals: the key role of cytochromes P450 reactivity. Trends Biochem Sci. 2020;45(6):511–25.
Sasaki J, Mikami A, Mizoue K, Omura S. Transformation of 25-and 1 alpha-hydroxyvitamin D3 to 1 alpha, 25-dihydroxyvitamin D3 by using Streptomyces sp. strains. Appl Environ Microbiol. 1991;57(10):2841–6.
Sasaki J, Miyazaki A, Saito M, Adachi T, Mizoue K, Hanada K, Omura S. Transformation of vitamin D3 to 1α, 25-dihydroxyvitamin D3 via 25-hydroxyvitamin D3 using Amycolata sp strains. Appl Microbiol Biotechnol. 1992;38(2):152–7.
Uchida E, Kagawa N, Sakaki T, Urushino N, Sawada N, Kamakura M, Ohta M, Kato S, Inouye K. Purification and characterization of mouse CYP27B1 overproduced by an Escherichia coli system coexpressing molecular chaperonins GroEL/ES. Biochem Biophys Res Commun. 2004;323(2):505–11.
Sakaki T, Akiyoshi-Shibata M, Yabusaki Y, Ohkawa H. Organella-targeted expression of rat liver cytochrome P450c27 in yeast. Genetically engineered alteration of mitochondrial P450 into a microsomal form creates a novel functional electron transport chain. J Biol Chem. 1992;267(23):16497–502.
Sawada N, Sakaki T, Kitanaka S, Takeyama K, Kato S, Inouye K. Enzymatic properties of human 25-hydroxyvitamin D3 1α-hydroxylase: coexpression with adrenodoxin and NADPH–adrenodoxin reductase in Escherichia coli. Eur J Biochem. 1999;265(3):950–6.
Hosseinpour F, Hidestrand M, Ingelman-Sundberg M, Wikvall K. The importance of residues in substrate recognition site 3 for the catalytic function of CYP2D25 (vitamin D 25-hydroxylase). Biochem Biophys Res Commun. 2001;288(4):1059–63.
Yamasaki T, Izumi S, Ide H, Ohyama Y. Identification of a novel rat microsomal vitamin D3 25-hydroxylase. J Biol Chem. 2004;279(22):22848–56.
Shinkyo R, Sakaki T, Kamakura M, Ohta M, Inouye K. Metabolism of vitamin D by human microsomal CYP2R1. Biochem Biophys Res Commun. 2004;324(1):451–7.
Sawada N, Sakaki T, Ohta M, Inouye K. Metabolism of vitamin D3 by human CYP27A1. Biochem Biophys Res Commun. 2000;273(3):977–84.
Blattner FR, Plunkett G, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF. The complete genome sequence of Escherichia coli K-12. Science. 1997;277(5331):1453–62.
Yamamoto K, Uchida E, Urushino N, Sakaki T, Kagawa N, Sawada N, Kamakura M, Kato S, Inouye K, Yamada S. Identification of the amino acid residue of CYP27B1 responsible for binding of 25-hydroxyvitamin D3 whose mutation causes vitamin D-dependent rickets type 1. J Biol Chem. 2005;280(34):30511–6.
Tang EK, Tieu EW, Tuckey RC. Expression of human CYP27B1 in Escherichia coli and characterization in phospholipid vesicles. FEBS J. 2012;279(19):3749–61.
Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci U S A. 2004;101(20):7711–5.
Strushkevich N, Usanov SA, Plotnikov AN, Jones G, Park H-W. Structural analysis of CYP2R1 in complex with vitamin D3. J Mol Biol. 2008;380(1):95–106.
Araya Z, Hosseinpour F, Bodin K, Wikvall K. Metabolism of 25-hydroxyvitamin D3 by microsomal and mitochondrial vitamin D3 25-hydroxylases (CYP2D25 and CYP27A1): a novel reaction by CYP27A1. Biochimica et Biophysica Acta BBA Mol Cell Biol Lipids. 2003;1632(13):40–7.
Aiba I, Yamasaki T, Shinki T, Izumi S, Yamamoto K, Yamada S, Terato H, Ide H, Ohyama Y. Characterization of rat and human CYP2J enzymes as vitamin D 25-hydroxylases. Steroids. 2006;71(10):849–56.
Rahmaniyan M, Patrick K, Bell NH. Characterization of recombinant CYP2C11: a vitamin D 25-hydroxylase and 24-hydroxylase. Am J Physiol Endocrinol Metab. 2005;288(4):e753-760.
Takeda K, Asou T, Matsuda A, Kimura K, Omura S. Application of cyclodextrin to microbial transformation of vitamin D3 to 25-hydroxyvitamin D3 and 1α,25-dihydroxyvitamin D3. J Ferment Bioeng. 1994;78(5):380–2.
Hayashi K, Yasuda K, Sugimoto H, Ikushiro S, Kamakura M, Kittaka A, Horst RL, Chen TC, Ohta M, Shiro Y. Three-step hydroxylation of vitamin D3 by a genetically engineered CYP105A1: enzymes and catalysis. FEBS J. 2010;277(19):3999–4009.
Kawauchi H, Sasaki J, Adachi T, Hanada K, Beppu T, Horinouchi S. Cloning and nucleotide sequence of a bacterial cytochrome P-450VD25 gene encoding vitamin D-3 25-hydroxylase. Biochimica et Biophysica Acta (BBA) Gene Struct Expr. 1994;1219(1):179–83.
Sawada N, Sakaki T, Yoneda S, Kusudo T, Shinkyo R, Ohta M, Inouye K. Conversion of vitamin D3 to 1α, 25-dihydroxyvitamin D3 by Streptomyces griseolus cytochrome P450SU-1. Biochem Biophys Res Commun. 2004;320(1):156–64.
Ang SS, Salleh AB, Chor LT, Normi YM, Tejo BA, Rahman MBA, Fatima M-A. Biochemical characterization of the cytochrome P450 CYP107CB2 from Bacillus lehensis G1. Protein J. 2018;37(2):180–93.
Urlacher VB, Girhard M. Cytochrome P450 monooxygenases in biotechnology and synthetic biology. Trends Biotechnol. 2019;37(8):882–97.
Zhang L, Wang Q. Harnessing P450 enzyme for biotechnology and synthetic biology. ChemBioChem. 2022;23(3): e202100439.
Sugimoto H, Shinkyo R, Hayashi K, Yoneda S, Yamada M, Kamakura M, Ikushiro S-I, Shiro Y, Sakaki T. Crystal structure of CYP105A1 (P450SU-1) in complex with 1α, 25-dihydroxyvitamin D3. Biochemistry. 2008;47(13):4017–27.
Hayashi K, Sugimoto H, Shinkyo R, Yamada M, Ikeda S, Ikushiro S, Kamakura M, Shiro Y, Sakaki T. Structure-based design of a highly active vitamin D hydroxylase from Streptomyces griseolus CYP105A1. Biochemistry. 2008;47(46):11964–72.
Ma B, Wang Q, Ikeda H, Zhang C, Xu L-H. Hydroxylation of steroids by a microbial substrate-promiscuous P450 cytochrome (CYP105D7): key arginine residues for rational design. Appl Environ Microbiol. 2019;85(23):e01530-e11519.
Fujii Y, Kabumoto H, Nishimura K, Fujii T, Yanai S, Takeda K, Tamura N, Arisawa A, Tamura T. Purification, characterization, and directed evolution study of a vitamin D3 hydroxylase from Pseudonocardia autotrophica. Biochem Biophys Res Commun. 2009;385(2):170–5.
Yasutake Y, Fujii Y, Nishioka T, Cheon W-K, Arisawa A, Tamura T. Structural evidence for enhancement of sequential vitamin D3 hydroxylation activities by directed evolution of cytochrome P450 vitamin D3 hydroxylase. J Biol Chem. 2010;285(41):31193–201.
Yasutake Y, Nishioka T, Imoto N, Tamura T. A single mutation at the ferredoxin binding site of P450 Vdh enables efficient biocatalytic production of 25-hydroxyvitamin D3. ChemBioChem. 2013;14(17):2284–91.
Ducharme J, Polic V, Auclair K. A covalently attached progesterone molecule outcompetes the binding of free progesterone at an allosteric site of cytochrome P450 3A4. Bioconjug Chem. 2019;30(6):1629–35.
Kawakami N, Shoji O, Watanabe Y. Use of perfluorocarboxylic acids to trick cytochrome P450BM3 into initiating the hydroxylation of gaseous alkanes. Angew Chem. 2011;123(23):5427–30.
Shoji O, Kunimatsu T, Kawakami N, Watanabe Y. Highly selective hydroxylation of benzene to phenol by wild-type cytochrome P450BM3 assisted by decoy molecules. Angew Chem Int Ed. 2013;52(26):6606–10.
Tataruch M, Heider J, Bryjak J, Nowak P, Knack D, Czerniak A, Liesiene J, Szaleniec M. Suitability of the hydrocarbon-hydroxylating molybdenum-enzyme ethylbenzene dehydrogenase for industrial chiral alcohol production. J Biotechnol. 2014;192:400–9.
Jacoby C, Eipper J, Warnke M, Tiedt O, Mergelsberg M, Stärk H-J, Daus B, Martín-Moldes Z, Zamarro MT, Díaz E. Four molybdenum-dependent steroid C-25 hydroxylases: heterologous overproduction, role in steroid degradation, and application for 25-hydroxyvitamin D3 synthesis. MBio. 2018;9(3):e00694-e1618.
Schmitz LM, Kinner A, Althoff K, Rosenthal K, Lütz S. Investigation of vitamin D2 and vitamin D3 hydroxylation by Kutzneria albida. ChemBioChem. 2021;22(13):2266.
Kang D-J, Im J-H, Kang J-H, Kim KH. Bioconversion of vitamin D3 to calcifediol by using resting cells of Pseudonocardia sp. Biotech Lett. 2015;37(9):1895–904.
Kang D-J, Im J-H, Kang J-H, Kim KH. Whole cell bioconversion of vitamin D3 to calcitriol using Pseudonocardia sp. KCTC 1029BP. Bioprocess Biosyst Eng. 2015;38(7):1281–90.
Tang D, Liu W, Huang L, Cheng L, Xu Z. Efficient biotransformation of vitamin D3 to 25-hydroxyvitamin D3 by a newly isolated Bacillus cereus strain. Appl Microbiol Biotechnol. 2020;104(2):765–74.
Takeda K, Kominato K, Sugita A, Iwasaki Y, Shimazaki M, Shimizu M. Isolation and identification of 2α, 25-dihydroxyvitamin D3, a new metabolite from Pseudonocardia autotrophica 100U–19 cells incubated with vitamin D3. Steroids. 2006;71(8):736–44.
Imoto N, Nishioka T, Tamura T. Permeabilization induced by lipid II-targeting lantibiotic nisin and its effect on the bioconversion of vitamin D3 to 25-hydroxyvitamin D3 by Rhodococcus erythropolis. Biochem Biophys Res Commun. 2011;405(3):393–8.
Sallam KI, Tamura N, Imoto N, Tamura T. New vector system for random, single-step integration of multiple copies of DNA into the Rhodococcus genome. Appl Environ Microbiol. 2010;76(8):2531–9.
Ban J-G, Kim H-B, Lee M-J, Anbu P, Kim E-S. Identification of a vitamin D3-specific hydroxylase genes through actinomycetes genome mining. J Ind Microbiol Biotechnol. 2014;41(2):265–73.
Abdulmughni A, Jóźwik IK, Brill E, Hannemann F, Thunnissen AMW, Bernhardt R. Biochemical and structural characterization of CYP109A2, a vitamin D3 25-hydroxylase from Bacillus megaterium. FEBS J. 2017;284(22):3881–94.
Abdulmughni A, Jóźwik IK, Putkaradze N, Brill E, Zapp J, Thunnissen A-MW, Hannemann F, Bernhardt R. Characterization of cytochrome P450 CYP109E1 from Bacillus megaterium as a novel vitamin D3 hydroxylase. J Biotechnol. 2017;243:38–47.
Abdulmughni A, Erichsen B, Hensel J, Hannemann F, Bernhardt R. Improvement of the 25-hydroxyvitamin D3 production in a CYP109A2-expressing Bacillus megaterium system. J Biotechnol. 2021;325:355–9.
Fujii T, Fujii Y, Machida K, Ochiai A, Ito M. Efficient biotransformations using Escherichia coli with tolC acrAB mutations expressing cytochrome P450 genes. Biosci Biotechnol Biochem. 2009;73(4):805–10.
Nakagawa A, Matsumura E, Koyanagi T, Katayama T, Kawano N, Yoshimatsu K, Yamamoto K, Kumagai H, Sato F, Minami H. Total biosynthesis of opiates by stepwise fermentation using engineered Escherichia coli. Nat Commun. 2016;7(1):1–8.
Quehl P, Schüürmann J, Hollender J, Jose J. Improving the activity of surface displayed cytochrome P450 enzymes by optimizing the outer membrane linker. Biochimica et Biophysica Acta (BBA) Biomembranes. 2017;1859(1):104–16.
Quehl P, Hollender J, Schüürmann J, Brossette T, Maas R, Jose J. Co-expression of active human cytochrome P450 1A2 and cytochrome P450 reductase on the cell surface of Escherichia coli. Microb Cell Fact. 2016;15(1):1–15.
Li S, Du L, Bernhardt R. Redox partners: function modulators of bacterial P450 enzymes. Trends Microbiol. 2020;28(6):445–54.
Meng X, Cao Q, Sun Y, Huang S, Liu X, Li D. 16S rRNA genes-and metagenome-based confirmation of syntrophic butyrate-oxidizing methanogenesis enriched in high butyrate loading. Biores Technol. 2022;345: 126483.
Morales-Contreras BE, Flórez-Fernández N, Torres MD, Domínguez H, Rodríguez-Jasso RM, Ruiz HA. Hydrothermal systems to obtain high value-added compounds from macroalgae for bioeconomy and biorefineries. Biores Technol. 2022;343: 126017.
Mussagy CU, Kurnia KA, Dias AC, Raghavan V, Santos-Ebinuma VC, Pessoa A Jr. An eco-friendly approach for the recovery of astaxanthin and β-carotene from Phaffia rhodozyma biomass using bio-based solvents. Biores Technol. 2022;345: 126555.
Luo J, Jiang F, Fang W, Lu Q. Optimization of bioconversion conditions for vitamin D3 to 25-hydroxyvitamin D using Pseudonocardia autotrophica CGMCC5098. Biocatal Biotransform. 2017;35(1):11–8.
Milhim M, Bernhardt R, Hannemann F, Gerber A, Neunzig J. A novel NADPH-dependent flavoprotein reductase from Bacillus megaterium acts as an efficient cytochrome P450 reductase. J Biotechnol. 2016;33:S212.
Acknowledgements
The authors would like to thank all the members of Zheng Lab and MARA Key Laboratory for their contributions on literature collection and critical reading of the manuscript.
Funding
This work was supported by the National Key R&D Program of China (2020YFA0906800), the National Natural Science Foundation of China (91851102 and 32070034), MARA Key Laboratory, China Animal Husbandry Industry Co., Ltd, and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA28030201).
Author information
Authors and Affiliations
Contributions
ZW, YZ (Yan Zeng) and YZ (Yanning Zheng) drafted the manuscript. HJ, NY, ML, MJ and YZ (Yanning Zheng) revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, Z., Zeng, Y., Jia, H. et al. Bioconversion of vitamin D3 to bioactive calcifediol and calcitriol as high-value compounds. Biotechnol Biofuels 15, 109 (2022). https://doi.org/10.1186/s13068-022-02209-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13068-022-02209-8