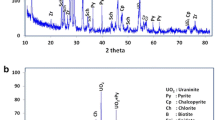
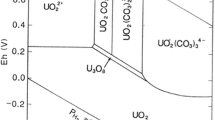
Abstract
Leaching of uranium and REEs with oxalic acid from the Egyptian Abu-Tartur phosphate rock (PR) was studied. The effect exerted on the leaching process by oxalic acid concentration, agitation time, solid to liquid ratio, and leaching temperature was determined. The leaching kinetics of uranium and REEs were evaluated by the shrinking core model. The uranium and REEs leaching kinetics is controlled by diffusion through the fluid film. The activation energy is 5.9 kJ/mol for U and 5.3 kJ/mol for REEs. Equations describing the leaching kinetics were obtained. The optimum conditions are oxalic acid concentration of 0.5 M, agitation time of 2 h, solid to liquid ratio of 1/4, and temperature of 70°C. Under these conditions, the degree of leaching of uranium and REEs reached 92 and 81%, respectively.








Similar content being viewed by others
REFERENCES
Sun, Y., Ding, Y.C., Cheng, W., and Wang, X., J. Hazard. Mater., 2014, vol. 280, pp. 399–408.
Edeltraud, H.R. and Rafat, W., Appl. Clay Sci., 2012, vols. 65–66, pp. 6–13.
Bigham, J.M. and Schulze, D., Iron oxides, Soil Mineralogy with Environmental Applications, Dixon, J.B., and Schulze, D.G., Eds., Madison: Soil Science Society of America Book Ser., 2002.
Zidan, I.H., Sedimentol. Egypt, 2013, vol. 2, pp. 55–67.
Gupta, C.K. and Singh, H., Uranium Resource Processing: Secondary Resources, Mumbai: Bhabha Atomic Research Centre, 2001.
Harvinderpal, S., Vijayalakshmi, R., Mishra, S.L., and Gupta, C.K., Hydrometallurgy, 2001, vol. 59, pp. 69–76.
Gharabaghi, M., Irannajad, M., and Noaparast, M., Hydrometallurgy, 2010, vol. 103, pp. 96–107.
Owens, C.L., Nash, G.R., Hadler, K., Fitzpatrick, R.S., Anderson, C.G., and Wall, F., Adv. Colloid Interface Sci., 2019, vol. 265, pp. 14–28. https://doi.org/10.1016/j.cis.2019.01.004
Sheng-xi, Wu, Long-sheng, Zhao, Liang-shi, Wang, Xiao-wei, Huang, Jin-shi, Dong, Zong-yu, Feng, Da-li, Cui, and Li-feng, Zhang, Trans. Nonferrous Met. Soc. China, 2018, vol. 28, pp. 2375−2382. https://doi.org/10.1016/S1003-6326(18)64883-6
Antonick, P.J., Hu, Z., Fujita, Y., Reed, D.W., Das, G., Wu, L., Shivaramaiah, R., Kim, P., Eslamimanesh, A., Lencka, M.M., Jiao, Y., Anderko, A., Navrotsky, A., and Riman, R.E., J. Chem. Thermodyn., 2019, vol. 132, pp. 491–496. https://doi.org/10.1016/j.jct.2018.12.034
Lan, X., Gao, J., Du, Y., and Guo, Z., Miner. Eng., 2019, vol. 133, pp. 27–34. https://doi.org/10.1016/j.mineng.2019.01.010
Picone, N., and Op den Camp, H.J., Curr. Opin. Chem. Biol., 2019, vol. 49, pp. 39–44. https://doi.org/10.1016/j.cbpa.2018.09.019
Yang, X., Werner, J., and Honaker, R.Q., J. Rare Earths, 2019, vol. 37, pp. 312–321. https://doi.org/10.1016/j.jre.2018.07.003
Sadeddin, W. and Abu-Eishah, S.I., Int. J. Miner. Process., 1990, vol. 30, pp. 113–125.
Abu-Eishah, S.I., Muthaker, M., Touqan, M. and Sadeddin, W., Miner. Eng., 1991, vol. 4, nos. 5/6, p. 573.
Zafar, Z.I., Anwar, M.M., and Pritchard, D.W., Nutrient Cycl. Agroecosyst., 1996, vol. 46, no. 2, p. 135.
Fredd, C.N. and Fogler, H.S., Chem. Eng. Sci., 1998, vol. 53, pp. 3863–3874.
Zafar, I.Z. and Saeed, A.K., J. Eng. Horizons, 2004, vol. 17, pp. 15–21.
Ashraf, M., Zafar, Z.I., and Ansari, T.M., Hydrometallurgy, 2005, vol. 80, pp. 286–292.
Zafar, Z.I. and Ashraf, M., Chem. Eng. J., 2007, vol. 131, p. 41.
Kpomblekou, A.K. and Tabatabai, M.A., Agr. Ecosyst. Environ., 2003, vol. 100, pp. 275–284.
Strobel, B.W., Geoderma, 2001, vol. 99, pp. 169–198.
Rodríguez, H. and Fraga, R., Biotechnol. Adv., 1999, vol. 17, pp. 319–339.
Sparks, D.L., Kinetics of Soil Chemical Processes, San Diego: Academic, 1989.
Kpomblekou, A.K. and Tabatabai, M.A., Soil Sci., 1994, vol. 158, pp. 442–453.
Sagoe, I.C., Ando, T., Kouno, K., and Nagaoka, T., Soil Sci. Plant Nutr., 1997, vol. 43, pp. 1067–1072.
Xu, R., Zhu, Y., and Chittleborough, D., J. Environ. Sci. China, 2004, vol. 16, no. 1, pp. 5–8.
Sengul, H., Ozer, A.K., and Gulaboglu, M.S., Chem. Eng. J., 2006, vol. 122, p. 135.
Farag, A.B., Bakry, A.R., Abdelfattah, N.A., and Elwy, A.M., Int. J. Adv. Res., 2015, vol. 3, no. 5, pp. 32–41.
Davies, W. and Gray, W., Talanta, 1964, vol. 11, no. 8, pp. 1203–1211.
Marczenko, Z., and Balcerzak, M., Separation, Preconcentration, and Spectrophotometry in Inorganic Analysis: Analytical Spectroscopy Library 10, Amsterdam: Elsevier, 2000.
Bassett, J., Denney, R.C., Jeffery, G.H., and Mendhan, J., Vogel’s Textbook of Quantitative Inorganic Analysis Including Elementary Instrumental Analysis, London: Longman, 1985,
Shapiro, L., and Brannock, W.W., Rapid Analysis of Silicate, Carbonate, and Phosphate Rocks, vol. 114A of US Geological Survey Bulletin, Washington: US Government, 1962.
Wang, L., Recovery of Rare Harths from Wet Process Phosphoric Acid, Presented at ICHM, Zhangjiajie (China), 2009.
Clauss, C.R.A. and Weiss, K. Adsorption of Aurocyanide on Carbon, Report of Investigation, Council for Scientific and Industrial Research, Pretoria, 1977, no. CENG 206.
Liddell, K.C., Hydrometallurgy, 2005, vol. 79, pp. 62–68.
Habashi, F., Principles of Extractive Metallurgy, General Principles, New York: Gordon and Breach, 1086.
Ray, H.S., Kinetics of Metallurgical Reactions, New Delhi: IBHC, 1993.
Rao, S.H., Yang, T., Zhang, D., Liu, W.F., Chen, L., and Hao, Z., Hydrometallurgy, 2015, vol. 158, pp. 101–106.
Bezzi, N., Aifa, T., Hamoudi, S., and Merabet, D., Procedia Eng., 2012, vol. 42, pp. 1915–1927.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors state that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Bakry, A.R., Hashim, M.D. & Elwy, A.M. Thermodynamic and Kinetic Studies of Uranium and REEs Leaching by Oxalic Acid from Abu-Tartur Phosphate Rock, Western Desert, Egypt. Radiochemistry 62, 359–367 (2020). https://doi.org/10.1134/S106636222003008X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S106636222003008X