Abstract
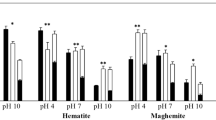
Sorption of U(VI) on natural and synthetic oxygen-containing minerals was studied. With respect to the sorption ability toward U(VI), natural minerals can be ranked in the order iron-containing > aluminum-containing > silicon-containing, and synthetic minerals, in the order iron-containing ≈ silicon-containing ≫ aluminum-containing. It was demonstrated by the example of Fe(OH)3, α-FeO(OH), and γ-FeO(OH) that crystalline minerals bind U(VI) at pH values corresponding to natural media (pH > 7.5) better than do amorphous minerals. The ionic strength does not affect the U(VI) sorption owing to formation of strong inner-sphere complexes on the mineral surface. The presence in solution of complexing agents in small amounts can enhance the U(VI) sorption owing to formation of ternary surface complexes, whereas large excess of the ligand decreases the sorption because of the shift of the equilibrium toward formation of soluble complexes migrating in the environment by long distances with water streams.
Similar content being viewed by others
References
Grenthe, I., Chemical Thermodynamics of Uranium, Paris: OECD, 2003.
Babkov, V.F. and Gerburt-Geibovich, A.V., Osnovy gruntovedeniya i mekhaniki gruntov (Principles of Soil Science and Soil Mechanics), Moscow: Vysshaya Shkola, 1964.
Missana, T., Garcia-Gutierrez, M., and Maffiotte, C., J. Colloid Interface Sci., 2003, vol. 260, pp. 291–301.
Zhang Hongxia, Xie Yongxin, and Tao Zuyi, Colloids Surf. A: Physicochem. Eng. Aspects, 2005, vol. 252, no. 1, pp. 1–5.
Hongxia, Z. and Zuyi, T., J. Radioanal. Nucl. Chem., 2002, vol. 254, no. 1, pp. 103–107.
Moyes, L.N., Parkman, R.H., Charnock, J.M., et al., Environ. Sci. Technol., 2000, vol. 34, no. 6, pp. 1062–1068.
Sherman, D.M., Peacock, C.L., and Hubbard, C.G., Geochim. Cosmochim. Acta, 2008, vol. 72, pp. 298–310.
Gabriel, U., Charlet, L., Schläpfer, C.W., et al., J. Colloid Interface Sci., 2001, vol. 239, pp. 358–368.
Chang, H.-S., Korshin, G.V., Wang, Z., and Zachara, J.M., Environ. Sci. Technol., 2006, vol. 40, no. 4, pp. 1244–1249.
Baumann, N., Brendler, V., Arnold, T., et al., J. Colloid Interface Sci., 2005, vol. 290, no. 2, pp. 318–324.
Um, W., Serne, R.J., Brown, C.F., and Rod, K.A., Appl. Geochem., 2008, vol. 23, pp. 2649–2657.
Keith-Roach, M.J., Sci. Total Environ., 2008, vol. 396, pp. 1–11.
Karyakin, Yu.V. and Angelov, I.I., Chistye khimicheskie veshchestva (Pure Chemicals), Moscow: Khimiya, 1974.
Brauer, G., Handbuch der präparativen anorganischen Chemie, Stuttgart: Enke, 1954.
Klyuchnikov, N.G., Rukovodstvo po neorganicheskomu sintezy (Manual on Inorganic Synthesis), Moscow: Khimiya, 1965.
Nemodruk, A.A. and Glukhova, L.P., Zh. Anal. Khim., 1963, vol. 43, no. 1, pp. 93–98.
Pshinko, G.N., Bogolepov, A.A., Kobets, S.A., and Kosorukov, A.A., Radiokhimiya, 2009, vol. 51, no. 2, pp. 187–190.
McKinley, J.P., Zachara, J.M., Smith, S.C., and Turner, G.D., Clays Clay Miner., 1995, vol. 43, no. 5, pp. 586–598.
Bargar, J.R., Reitmeyer, R., Lenhart, J.J., and Davis, J.A., Geochim. Cosmochim. Acta, 2000, vol. 64, no. 16, pp. 2737–2749.
Froideval, A., Del Nero, M., Barillon, R., et al., J. Colloid Interface Sci., 2003, vol. 266, no. 1, pp. 221–235.
Kornilovich, B.Yu., Pshinko, G.N., and Bogolepov, A.A., Radiokhimiya, 2006, vol. 48, no. 6, pp. 525–528.
Nowack, B., Lützenkirchen, J., Behra, P., and Sigg, L., Environ. Sci. Technol., 1996, vol. 30, no. 7, pp. 2397–2405.
Nowack, B. and Sigg, L., J. Colloid Interface Sci., 1996, vol. 177, no. 1, pp. 106–121.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.V. Goncharuk, G.N. Pshinko, S.A. Kobets, A.A. Kosorukov, A.A. Bogolepov, 2010, published in Radiokhimiya, 2010, Vol. 52, No. 3, pp. 241–246.
Rights and permissions
About this article
Cite this article
Goncharuk, V.V., Pshinko, G.N., Kobets, S.A. et al. Influence of the nature of oxygen-containing minerals on their sorption ability toward U(VI). Radiochemistry 52, 284–290 (2010). https://doi.org/10.1134/S1066362210030112
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1066362210030112