Abstract
Soil organic matter (SOM) includes many classes of labile compounds available for microbial decomposition or, conversely, stable compounds protected from biodegradation by biological, chemical, and physical stabilization. It is believed that the more thermal energy is spent on the destruction of soil organic matter, the more stable and more resistant for biodegradation it is. We compared the thermal and biological stabilities of organic matter in eleven soil types from deciduous forest, forest-steppe, steppe, and semidesert bioclimatic areas of the European Russia. According to the activation energy (Ea), the highest SOM thermal stability was typical of the ordinary chernozem and meadow vertic soil. The lowest SOM thermal stability was found for gray forest soil; other soil types were characterized by an intermediate resistance towards thermal oxidation. The thermally labile pool (<390–400°C) of organic matter in soils was on the average 41% (32–60%) of the total SOM, while the thermally stable pool (>390–400°C) was on the average 59% (40–68%). The SOM biological stability estimated by the ratio of potentially mineralizable organic matter to that resistant to mineralization (biological stability index) decreased in the following order: ordinary chernozem (Haplic Chernozem (Loamic, Pachic)) > meadow vertic soil (Pellic Vertisol (Gleyic, Humic)) > gray forest soil (Luvic Greyzemic Phaeozem (Loamic)) = meadow chestnut soil (Gleyic Kastanozem (Chromic)) > meadow solonetz (Endosalic Gleyic Solonetz (Loamic, Cutanic)) > alluvial meadow soil (Eutric Fluvisol (Humic, Oxyaquic)). The potentially mineralizable SOM pool in the studied soils was 6–27-fold lower as compared with the pool of thermally labile SOM, and the parameters that characterize SOM thermal stability did not correlate with the biological stability index. Thus, SOM thermal lability is not identical to its biodegradability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Soil is a complex self-regulating multicomponent system represented by solid, liquid, gas, and living phases. The interaction between phases forms the background of many phenomena and effects underlying soil similarity as well as variety of properties and regimes. Particulate matter representing the remains of biota, nonhumic biomolecules, and ensembles of humic substances stochastically adsorbed in a conglomerate of mineral particles constitute the solid part of soil organic matter (SOM) [5]. The biota remains and the products of microbial metabolism forming SOM have different initial and secondary chemical stabilities [9, 20, 24]. The initial stability of organic matter is determined by the properties of its constituent compounds that which differ in the ratio of elements, forms of molecules, and composition of functional groups. The most chemically stable compounds resistant to decomposition are lignin, tannin, cutin, suberin, and waxes. The secondary stability of organic matter emerges from its biological and chemical transformation in soil as a result of an increase in the share of lignin and polyphenols in the decomposed residues, synthesis of microbial metabolites of melanin and glomalin type, formation of humic substances, and charring [9]. It is traditionally believed that the higher the share of stable organic compounds in SOM, the higher is its stability [3, 9, 14, 29, 30, 46].
According to the current concept, chemical stability of compounds is responsible for short-term and middle-term protection of organic matter over several years to several decades, while a long-term preservation of SOM components over several hundreds and thousands of years is provided by stabilization of organic compounds by the mineral part of soil [3, 25, 45, 46]. The mineral part of solid phase catalyzes condensation and polymerization reactions, sorbs biomolecules of organic monomers, contributes to formation of organometallic and coordination bonds, and creates physical barriers between microorganisms (enzymes) and substrates, making the latter spatially unavailable. The remains of microbial biomass are stabilized by the surface of mineral particles faster and stronger as compared with the particulate organic matter of plant residues and the monomers, faster and stronger than large molecules of biopolymers [18, 19]. Correspondingly, the organic compounds even simple in their chemical composition but bound by soil mineral matrix reflect a high resistance and pronounced stability.
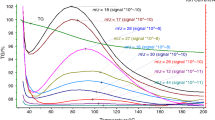
Derivatographic analysis makes it possible to assess the ratio of different-phase soil components as well as the strengths of internal and external bonds in SOM. This method records chemical and physicochemical processes taking place with a change in temperature (phase transition, thermal decomposition and oxidation, intramolecular rearrangements, etc.) to get the curves of four types: thermal (T), differential thermal (DTA), thermogravimetric (TG), and differential thermogravimetric (DTG) [4]. The DTA curve characterizes the phase transition in a sample and the TG curve demonstrates the change in sample weight under nonisothermal and isothermal conditions, giving the insight into thermal (thermal oxidative) stability of soil components. The local peaks in the DTG curve are explainable with a change in the decomposition mechanisms and the composition of decomposed material. TG and DTG curves are used to assess the SOM qualitative composition, to determine the ratio of its thermally labile and stable pools, to study the structure of supramolecular complexes, and to identify pyrogenic, organic, and inorganic carbon in soil [2, 12, 15–17, 21, 27, 31, 38, 39, 43, 44].
There are several temperature intervals with similar patterns of soil weight loss corresponding to the evaporation of adsorption-bound water, organic matter decomposition, and physicochemical transformations of soil minerals [1, 2, 15–17, 21, 26, 27, 31, 32, 35, 39, 44]. According to the degree of resistance to thermal destruction, the overall SOM is divided into the labile pool with weakly bound biologically degradable organic matter and the stable pool with tightly bound, mainly humic components [2, 26, 33, 39]. Analytical pyrolysis and thermogravimetry in combination with differential scanning calorimetry, mass spectrometry, or chromatography–mass spectrometry identify three group of organic substances differing in their stability to thermal destruction, namely, labile, recalcitrant, and refractory [13, 16, 21, 22, 26, 27, 31, 36]. The temperature ranges characterizing thermal stability of these three groups may vary depending on the research conditions and soil properties [42].
According to the earlier concept, thermolabile organic matter is more biodegradable as compared with the thermostable matter; correspondingly, the thermal properties of individual SOM groups can act as indicators of their biological decomposition by soil microorganisms [28, 34, 38, 39]. However, some studies have given other results. The soil samples with low and high Corg contents reflect different correlations between the thermal and biological stabilities of SOM [33]. The SOM pools differing in their thermostability have emerged to be nonidentical to the biologically active SOM pool, while the thermal oxidation failed in general to identify the SOM pools differing in their biological stability [21, 37]. It was not possible to isolate biologically stable SOM fractions bound to soil minerals by increasing temperature from 200 to 500°C [23].
The goal of this work was to compare the sizes of thermolabile, thermostable, and biologically active SOM pools isolated by derivatographic and biokinetic methods for the soils differing in their particle size distribution and humus-forming conditions and to clarify the degree to which the SOM thermostability characterizes its biological stability.
OBJECTS AND METHODS
Characterization of the studied soils. Samples of different soil types taken from under natural vegetation and agricultural crops in the deciduous forest, forest-steppe, steppe, and semidesert bioclimatic regions of the European Russia were used in the study. Soil was sampled from the upper humus horizon from three walls of soil profiles made for educational purposes. The sites of natural and arable lands resided within the same studied area. The freshly collected samples were immediately air-dried in the open air. Table 1 lists the soil names, sampling sites, land use, contents of organic carbon (Corg) and nitrogen (Ntot) in soil, and characteristics of particle size distribution.
Derivatographic analysis of soils. A Paulik–Paulik–Erdey Q-1500D (Hungary) derivatograph comprises a unit for differential thermal analysis and a thermobalance for thermogravimetry. The DTA unit has a differential temperature (ΔT between the tested sample and standard, Al2O3) recorder and a heating temperature (T) recorder. The unit is heated with a programmed controller guaranteeing a specified heating rate and a uniform temperature increase. Platinum crucibles with thermowells are filled with soil and reference substance. The thermowells are connected to mirror galvanometers; a furnace is used for heating. The corresponding blocks record the signals and upload them to a PC [1].
All visible plant residues were removed from air-dry soil samples; soil samples (5–10 g) were ground in an agate mortar to a size of <0.05 mm. Each weighed sample (0.5 g) was placed into a platinum crucible, capped, transferred to the sample holder of DTA unit, covered with a quartz cylinder, and placed into a furnace. The heating rate was 10°/min and final temperature, 1000°C. The recorded thermograms (Figs. 1 and S1) were marked for further calculation of the relative weight loss corresponding to the maximum rate of thermal decomposition. The DTG curve in the marked thermogram was used to determine the beginning and end of weight loss (a single-stage process). The values of the corresponding temperatures of the start (Ts) and end (Te) of the stage were plotted on the TG curve to find the temperature interval of the reaction (Ts – Te). The thermal decomposition of organic matter was regarded as a sequence of single-stage processes represented by single peaks. The DTA curve was used to assess the character of peaks (exothermal or endothermal) and to calculate the width, height, area, and the extrapolated point where the process started. The exotherms and endotherms with a poor resolution were explained by overlapping of thermal decomposition processes. The oxidation of thermal decomposition products gives a pronounced exothermal peak [1]. The TG curve was used to determine the rate of thermal decomposition at a specified temperature or the maximum rate of decomposition corresponding to the minimum in the DTG curve as well as to calculate the activation energy of thermal destruction (Ea) by the Reich–Fuoss method using Simulink software (MatLab).
Assessing SOM mineralization capacity and biological stability. The potentially mineralizable organic matter was assessed by incubating intact soil samples (10 g) at constant temperature (22°C) and moisture content (25 wt %) with C–CO2 quantification over 160–170 days, as described by Semenov et al. [6]. Soil samples were incubated in glass 100-mL flasks in triplicate. The C–CO2 concentration in the gas phase of incubated samples was first measured after 3–4 h; during the first week, on a daily basis; then, three times per week; during the second month of incubation, two times per week; starting from the third month of incubation, one time per week. After each measurement, the flasks were aired out. The C–CO2 concentration was determined in a KristalLux 4000 M gas chromatograph to determine the flow rate (mg/(100 g per day), cumulative C–CO2 production (mg/100 g), and the carbon content of active (potentially mineralizable) SOM by the beginning of incubation, calculated using the following one-component equation of the first-order kinetics:
where Ct is the cumulative C–CO2 amount (mg/100 g soil) over time t; C0 is the content of active (potentially mineralizable) carbon (mg/100 g); and k, mineralization rate constant (day–1).
The cumulative curves of C–CO2 production are shown in Fig. S2. Biokinetic parameters C0 and k were fitted by nonlinear estimation (Statistica software package) and the biological stability of SOM was assessed by the corresponding biological stability index (BSI) as
It is assumed that the higher the ratio of the carbon content in the stable pool to that in the active pool, the more stable is the organic matter in soil.
Determining physicochemical properties of soil. The total carbon and total nitrogen contents in soil samples were determined in a CNHS analyzer (Leco, United States). The content of organic carbon (Corg) was determined by subtracting the carbonate content from the total carbon content. The carbonates in soil were assessed by acidimetric technique; cation exchange capacity, according to the Bobko–Askinazi method in the CINAO modification; \({\text{p}}{{{\text{H}}}_{{{{{\text{H}}}_{{\text{2}}}}{\text{O}}}}}\), with PB-11 (Sartorius Basic Meter, United States) pH-meter; and particle-size distribution, by pipette method with sodium pyrophosphate treatment [10, 11]. Table 1 lists the contents of particles of >0.01 and <0.001 mm.
RESULTS AND DISCUSSION
Derivatographic determination of SOM content and stability. The recorded thermograms (Figs. 1 and S1) were used to determine the composition of the components in the studied soils. The mineral part of the solid phase in humus horizons accounts for 82–96 wt % and organic part, for 3.3–15.0 wt % of (Table 2). The percentage of adsorption-bound water varied from 1.0 to 3.4 wt % of the air-dry samples. The lowest ratio of mineral mass to organic matter is observed in the ordinary chernozem and meadow vertic soil (5 to 8) and the highest ratio, in the arable gray forest soil and the lower layers of humus horizon in the meadow solonetz and alluvial meadow soil (20 to 29). Different ratios of the mineral to organic parts in the soil solid phase are mainly determined by the variation in SOM content. The variation coefficient for the mineral part of the studied soils amounted to 4% versus 38% for the organic part. The variation of soil mineral part was mainly determined by the content of physical sand (r = 0.750, P = 0.001) and of the organic part, by the content of physical clay (r = 0.653, P = 0.006). The SOM content in the particle size fractions belonging to physical clay correlated with the percentage of the <0.001 mm particles in a statistically significant manner.
The thermal and thermogravimetric effects recorded by derivatograph give the insight into the character and effectiveness of the chemical and physicochemical transformations in a soil sample and the resulting change in the sample weight. The total weight losses during a gradual heating of soil sample to 1000°C are composed of water evaporation, SOM combustion, and unidentified losses (Table 3). The percentage of water in thermal destruction losses was 14–30%; of SOM, 49–83%; and of unidentified material, most likely represented by carbonates and unstable fragments of soil minerals, from 1 to 26%.
The temperature of water desorption and evaporation on the average amounted to 122°C, varying from 100 to 137°C for different soil types. The thermal destruction of SOM took place in the temperature interval from 179–301 to 484–890°C and the unidentified losses, at temperatures over 484–890°C. The higher the upper boundary of SOM thermal destruction, the smaller amount of matter accounted for unidentified losses (r = –0.924, P < 0.001). Other studies reported the water elimination from soil at a temperature ranging from 25–40 to 150–220°C; SOM thermal destruction, from 180–200 to 550–700°C; and thermal destruction of individual minerals, mainly from 600–700 to 900–1000°C [4, 15, 16, 21, 26, 27, 31, 32, 35, 39, 44].
The minimum amount of thermal energy necessary for chemical and physicochemical reactions is referred to as activation energy (Ea). The smaller the Ea value, the higher is the rate of the reaction initiated by an increase in temperature and the less stable are the substances. A complex SOM heterogeneous in its composition is decomposed in several stages differing in their rates and, consequently, in the activation energies. It is believed, the energy necessary for the overall thermal destruction process is controlled by its slowest stage, which requires the highest activation energy. That is why the Ea value directly depends on both the number of components and the strength of internal and external bonds. The larger the amount of organic matter in a soil sample and the smaller the share of its mineral part, the higher is the activation energy (Fig. 2). On the other hand, an inverse correlation between Ea and unidentified substances (r = –0.751, P = 0.001) and the absence of any significant correlation with the amount of organic matter and water in the thermal destruction losses suggest that the unidentified losses are mainly caused by the destruction of some soil minerals and that they are responsible for the main expenditures of thermal energy.
According to the Ea values, the highest thermal stability is characteristic of the SOM of ordinary chernozem and meadow vertic soil and the lowest, 3.3-fold smaller as compared with chernozem, of the organic matter of gray forest soil (Table 3). The remaining soil types form the group of intermediate thermal stability. The energy spent for SOM oxidation in the samples of the upper layer in humus horizon was 1.1–2.9-fold higher as compared with the samples of lower layers independently of the soil type. The differences in the Ea value between the layers within the humus horizon are most likely associated with that the upper layer SOM is enriched with thermostable compounds associated with the particulate organic matter (POM), formed during decomposition of plant residues. As shown earlier [8], the POM weight together with the sand-size fraction is 1.2–1.4 times higher as compared with the lower layer. Thus, the Ea value is indicative of the SOM thermal stability.
Analysis of the DTG curves for the studied soils shows several peaks suggesting that the organic compounds of SOM have different thermal sensitivities. The known classes of organic compounds have different sensitivities to thermal impacts depending on their chemical composition, presence of aliphatic and aromatic structures, and other properties; they fall into two or three groups according to thermal stability. The group of easily combustible carbon (300°C) comprises mainly aliphatic compounds with carboxyl groups and the group of difficultly combustible carbon (450°C), mainly aromatic compounds [39]. Three groups were distinguished according to thermogravimetry in combination with differential scanning calorimetry: (i) labile organic substances (200–380°C), manly carbohydrates and other aliphatic compounds; (ii) recalcitrant substances (380–475°C), represented by lignin and polyphenols; and (iii) refractory (475–650), polycondensed aromatic compounds including black carbon [31]. Carbohydrates, peptides, phenols, lignin monomers, and the other compounds of the light density fraction (<2.0 g/cm3), represented by partially decomposed plant residues, are more thermolabile as compared with the same compounds of the heavy fraction (>2.2 g/cm3), while the mineral-bound organic compounds have the highest stability [38]. Selection of the temperature ranges where the SOM is regarded as thermostable or thermolabile is rather arbitrary, depends on the experimental conditions, and must be based on the specific features of TG or DTG curves in each particular case [44].
Consequently, the natural thermal stability of organic compounds can change depending on the type of their stabilization in soil. Presumably, a compound simple in its chemical composition and structure but stabilized in soil is more thermostable as compared with a more complicated compound of aromatic structure but chemically or physically unprotected. That is why, SOM derivatographic analysis gives an integrated view of the nature of SOM and its protection. The largest number of peaks in DTG curves, which is observable in the samples of arable chernozem, is determined by the diversity of mechanisms involved in stabilization of organic compounds in soil rather than by a wide range of SOM components (Table 4). In the low temperature region with thermolabile SOM, 15 samples of the 16 analyzed had only one peak versus the high temperature region with stable SOM, where seven samples had two peaks; another seven samples, three peaks, and one more sample, even four peaks. In the studied soils, 32 to 60% of the SOM may be regarded as belonging to thermolabile pool and 40 to 68%, respectively, to thermostable pool. The SOM thermolabile pool was larger than the thermostable one only in one sample of the lower humic horizon layer in meadow-bog soil; two samples had the pools of approximately the same size; and thermostable components were prevalent in the remaining samples (this was especially pronounced in chernozem, meadow vertic soil, and chestnut solonetz). On the average for different soil types, the thermolabile and thermostable pools contained 41 and 59% of SOM. Note that the SOM thermolabile pool in the gray forest soil of the Tula oblast (40–52%) was slightly larger as compared with the labile pool of the same soil in the Moscow oblast (32–42%) assessed using 13C isotope [3]. Presumably, thermal decomposition affects a larger SOM amount as compared with the SOM able to be recycled.
Another peculiarity is a wide variation range of the ratio of the SOM weight according to derivatography and the Corg content determined by dry combustion (1.83 to 6.58) although these characteristics correlate in a statistically significant manner (r = 0.836, P < 0.001). Correspondingly, it is incorrect to use some universal coefficients for converting Corg to organic matter or humus.
Biological stability of organic matter in different soils. The biological stability of SOM is defined as the resistance to microbial decomposition and mineralization arising from chemical, physical, and biological stabilization of the organic compounds in the soil. The organic matter in soil acquires protection and stability due to formation of humic substances and organomineral complexes, physical barriers and spatial inaccessibility resulting from aggregation, initial (or acquired) recalcitrance of organic compounds, deactivation of enzymes, and an uncomfortable medium for microorganisms [9, 25, 41, 45, 46]. The available SOM potentially mineralizable by microorganisms is regarded as biologically active and the stable and poorly biodegradable SOM, as biologically stable [7]. The biological stability of SOM is quantitatively illustrated by three parameters: (i) percentage of active organic matter in the total SOM content; (ii) mineralization rate constant; and (iii) BSI [6].
The humus horizon of uncultivated zonal soils (gray forest soil, ordinary chernozem, meadow chestnut soil, and chestnut solonetz) contained 92–131 mg/100 g of active SOM carbon (Table 5). Characteristic of the arable analogs of gray forest soil and ordinary chernozem is a considerable decrease of the active SOM in their humus horizon. The surface layer (0–2 to 0–8 cm) of the humus horizon in intrazonal soils contained 1.4–4.9-fold more active SOM (108–212 mg/100 g) as compared with the adjacent layers at a depth down to 15–30 cm from the soil surface (23–199 mg/100 g). According to these data, only 1.9 to 7.5% of Corg was biologically active (Table 5). The SOM in the lower layers of the humus horizons in intrazonal soils had a lower mineralization capacity as compared with the surface layers (2.2–5.1 and 4.0–7.5% of Corg, respectively) and the SOM of the arable gray forest soil and chernozem, as compared with uncultivated analogs (1.9–3.2 and 2.7–4.2% of Corg, respectively).
The SOM mineralization constants in the upper horizon of zonal and intrazonal soils were of the same order of magnitude (0.013–0.043 day–1) and almost did not depend on either soil properties or changes in land use. This suggests that the potentially mineralizable SOM pools in zonal and intrazonal soils are represented by approximately the same set of organic substances, while certain differences between soils are determined by external and internal factors that control the availability of organic components to soil microorganisms and the strength of stabilization of the remains of biota they transform.
The BSI values of SOM were determined; these indices are the factor showing the degree to which the amount of carbon resistant to mineralization is larger than the amount of potentially mineralizable one. The BSI value decreased in the following order: ordinary chernozem > meadow vertic soil = meadow-bog soil > gray forest soil = meadow chestnut soil > meadow solonetz > chestnut solonetz = steppe solonetz = alluvial meadow soil. Presumably, an increased biological stability of the organic matter in, for example, meadow-bog soil is determined by a weak growth of microbial biomass because of the nutrition and living conditions unfavorable for microorganisms and in the case of chernozems, by a rapid and complete stabilization of microbial biomass and decomposition products. Thus, determination of potentially mineralizable organic matter in a long-term incubation of soil samples with quantification of the evolved C–CO2 is the most reliable method for assessing the biological stability of SOM. Although the stock of organic matter in soils is large enough, only a small SOM part is available to microorganisms and can be mineralized over the warm season of the year.
Comparison of SOM thermal and biological stabilities. It is assumed that the SOM components destructed at relatively low combustion temperatures are better available to microorganisms and more suitable as a source of energy and nutrition as compared with the organic substances degradable at higher temperatures [2, 12, 34, 40]. The thermal stability of SOM matches best to its biodegradability in the low temperature region of <350°C [34]. The soil weight losses measured by thermogravimetry at a temperature of 260°C positively correlated with the CO2 emission during the incubation of soil samples [40]. However, the update demonstrate different correlations between SOM thermal and biological stabilities for the samples with a low and a high Corg content in soil, which is determined by different mechanisms responsible for SOM stabilization [33]. According to other studies, the amount of combusted organic matter weakly depended on the increase in temperature in the range of 200–400°C and was comparable with neither labile (particulate organic matter) nor stable (mineral-associated organic matter) SOM [37]. The mean residence time (MRT) of carbon was almost the same for three SOM fractions with destruction temperatures of 190–310, 310–390, and 390–480°C (11.6, 12.2, and 15.4 years, respectively) versus the SOM pool degradable at 480–1000°C, where the MRT was 163 years [21]. Correspondingly, although the SOM comprises thermolabile and thermostable pools, the thermal lability and biodegradability of SOM are poorly correlated with one another. It is also shown that the SOM fraction resistant to thermal oxidation at 300°C contained a considerable amount of “young” carbon that came to soil from corn and that the thermal oxidation with the temperature increase from 200 to 500°C was unsuitable for isolation of the mineral-bound SOM fractions [23].
In this study, we have not found any statistically significant correlation of the activation energy (Ea) with the content of potentially mineralizable organic matter (C0), percentage of C0 in Corg, mineralization rate constant, and BSI. Paradoxically, the size of thermostable pool positively correlated with the content of potentially mineralizable organic matter, whereas any correlations with the other parameters of biologically active pool, including BSI, were statistically insignificant. Correspondingly, thermal energy is not identical to the energy of enzymatic reactions in soil and thermal oxidation of organic matter is not identical to biological oxidation, catalyzed by enzymes. Thermal and biokinetic analyses give different and unrelated characteristics of SOM quality and the prevalent mechanisms ensuring SOM stability. The thermolabile organic matter most likely comprises the components of SOM active and slow pools and the thermostable organic matter, of SOM slow and passive pools.
CONCLUSIONS
The stability of SOM is the function of soil physicochemical properties, biological activity of soil communities, and environmental factors and is one of the main SOM properties that guarantee its long-term preservation. Different ways and mechanisms of the organic matter stabilization and destabilization in soil render SOM physically, chemically, and biologically stable, which is assessed with the corresponding fractionation techniques.
The thermal and thermogravimetric effects during a gradual heating of soil sample to 1000°C give the insight into the thermal (thermal oxidative) stability of SOM and the qualitative composition of its components. According to the activation energy—the minimum amount of thermal energy necessary for chemical and physicochemical reactions—the SOM of ordinary chernozem and meadow vertic soil has the highest thermostability and the SOM of gray forest soil, the lowest. The remaining studied soil types are intermediate in terms of their thermal stability.
The SOM susceptible to thermal destruction in a low temperature range (<390–400°C) is regarded as thermolabile and the SOM oxidized in a high temperature range (>390–400°C), as thermostable. On the average, the thermolabile and thermostable pools of different soil types contained 41 and 59% of organic matter, respectively. The differential thermogravimetric curves of the studied soils had two to five peaks, suggesting SOM heterogeneity and a variety of mechanisms underlying their thermal stability.
The intensity of C–CO2 production is both the qualitative and quantitative characteristic of soil mineralization capacity and the ratio of the organic matter resistant to mineralization to the potentially mineralizable organic matter is the measure of its biological stability. The biological stability of organic matter in the examined soils decreased in the following order: ordinary chernozem > meadow vertic soil > gray forest soil > meadow solonetz > alluvial meadow soil. A decrease in the active organic matter in arable soils is accompanied by an increase in the share of stable and biologically conservative organic matter.
The thermal lability of SOM is not identical to its capability for biodegradation, while thermal and biokinetic analyses give different and unrelated characteristics of the SOM quality and the prevalent mechanisms providing its stability.
Change history
28 July 2021
An Erratum to this paper has been published: https://doi.org/10.1134/S1064229321770015
REFERENCES
S. L. Belopukhov, T. V. Shnee, I. I. Dmitrievskaya, M. D. Maslova, E. A. Grihsina, and E. V. Kalabashkina, Methodological Guidelines for Testing Biological Samples by Thermal Analysis (Timiryazev Agricultural Academy, Moscow, 2014) [in Russian].
A. A. Bolatov, V. A. Chernikov, and S. M. Lukin, “Derivatographic analysis of the humus state in soddy-podzolic sandy loamy soils,” Agrokhim. Vestn., No. 3, 38–40 (2010).
A. A. Larionova, B. N. Zolotareva, I. V. Yevdokimov, S. S. Bykhovets, Ya. V. Kuzyakov, and F. Buegger, “Identification of labile and stable pools of organic matter in an agrogray soil,” Eurasian Soil Sci. 44, 628–640 (2011).
I. A. Makarova and N. A. Lokhova, Physicochemical Methods of the Analysis of Construction Materials (Bratsk State Univ., Bratsk, 2011) [in Russian].
V. M. Semenov and B. M. Kogut, Soil Organic Matter (GEOS, Moscow, 2015) [in Russian].
V. M. Semenov, B. M. Kogut, N. B. Zinyakova, N. P. Masyutenko, L. S. Malyukova, T. N. Lebedeva, and A. S. Tulina, “Biologically active organic matter in soils of European Russia,” Eurasian Soil Sci. 51, 434–447 (2018). https://doi.org/10.1134/S1064229318040117
V. M. Semenov, I. K. Kravchenko, L. A. Ivannikova, T. V. Kuznetsova, N. A. Semenova, M. Gispert, and J. Pardini, “Experimental determination of the active organic matter content in some soils of natural and agricultural ecosystems,” Eurasian Soil Sci. 39, 251–260 (2006).
V. M. Semenov, T. N. Lebedeva, and N. B. Pautova, “Particulate organic matter in noncultivated and arable soils,” Eurasian Soil Sci. 52, 396–404 (2019). https://doi.org/10.1134/S1064229319040136
V. M. Semenov, A. S. Tulina, N. A. Semenova, and L. A. Ivannikova, “Humification and nonhumification pathways of the organic matter stabilization in soil: a review,” Eurasian Soil Sci. 46, 355–368 (2013).
Theory and Methods of Soil Physics: Monograph, Ed. by E. V. Shein and L. O. Karpachevskii (Grif i K, Moscow, 2007) [in Russian].
Theory and Practice of Chemical Analysis of Soils, Ed. by L. A. Vorob’eva (GEOS, Moscow, 2006) [in Russian].
N. V. Uskova, V. A. Chernikov, and S. L. Belopukhov, “Agroecological assessment of the effect of long-term application of fertilizers on the humus state of soddy-podzolic soil,” Izv. Timiryazevsk. S-kh. Akad., No. 2, 18–33 (2018). https://doi.org/10.26897/0021-342X-2018-2-18-33
V. A. Kholodov, Yu. R. Farkhodov, A. Ya. Zherebker, and N. V. Yaroslavtseva, “Possible use of analytical two-stage pyrolysis with gas chromatography-mass spectrometry for the study of humic substances in situ,” Byull. Pochv. Inst. im. V.V. Dokuchaeva, No. 94, 3–18 (2018). https://doi.org/10.19047/0136-1694-2018-94-3-18
S. N. Chukov, E. D. Lodygin, and E. V. Abakumov, “Application of 13C NMR spectroscopy to the study of soil organic matter: a review of publications,” Eurasian Soil Sci. 51, 889–900 (2018). https://doi.org/10.1134/S1064229318080021
O. A. Shapchenkova, A. A. Aniskina, and S. R. Loskutov, “Thermal analysis of organic matter in cryogenic soils (Central Siberian Plateau),” Eurasian Soil Sci. 44, 399–406 (2011).
O. A. Shapchenkova, Yu. N. Krasnoshchekov, and S. R. Loskutov, “Application of the methods of thermal analysis for the assessment of organic matter in postpyrogenic soils,” Eurasian Soil Sci. 44, 677–685 (2011).
P. Boguta, Z. Sokołowska, and K. Skic, “Use of thermal analysis coupled with differential scanning calorimetry, quadrupole mass spectrometry and infrared spectroscopy (TG-DSC-QMS-FTIR) to monitor chemical properties and thermal stability of fulvic and humic acids,” PLoS One 12, e0189653 (2017). https://doi.org/10.1371/journal.pone.0189653
M. F. Cotrufo, J. L. Soong, A. J. Horton, E. E. Campbell, M. L. Haddix, D. H. Wall, and W. J. Parton, “Formation of soil organic matter via biochemical and physical pathways of litter mass loss,” Nat. Geosci. 8, 776–779 (2015). https://doi.org/10.1038/NGEO2520
M. F. Cotrufo, M. D. Wallenstein, C. M. Boot, K. Denef, and E. Paul, “The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant in-puts form stable soil organic matter?” Global Change Biol. 19 (4), 988–995 (2013). https://doi.org/10.1111/gcb.12113
S. Derenne and C. Largeau, “A review of some important families of refractory macromolecules: composition, origin, and fate in soil and sediments,” Soil Sci. 166 (11), 833–847 (2001). https://doi.org/10.1097/00010694-200111000-00008
M. Dorodnikov, A. Fangmeier, and Y. Kuzyakov, “Thermal stability of soil organic matter pools and their δ13C values after C3–C4 vegetation change,” Soil Biol. Biochem. 39 (5), 1173–1180 (2007). https://doi.org/10.1016/j.soilbio.2006.12.025
F. Guo, F. Wu, Y. Mu, Y. Hu, X. Zhao, W. Meng, J. P. Giesy, and Y. Lin, “Characterization of organic matter of plants from lakes by thermal analysis in a N2 atmosphere,” Sci. Rep. 6 (22877) (2016). https://doi.org/10.1038/srep22877
M. Helfrich, H. Flessa, A. Dreves, and B. Ludwig, “Is thermal oxidation at different temperatures suitable to isolate soil organic carbon fractions with different turnover?” J. Plant Nutr. Soil Sci. 173 (1), 61–66 (2010). https://doi.org/10.1002/jpln.200700280
I. Kögel-Knabner, “The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter,” Soil Biol. Biochem. 34 (2), 139–162 (2002). https://doi.org/10.1016/S0038-0717(01)00158-4
I. Kögel-Knabner, K. Ekschmitt, H. Flessa, G. Guggenberger, E. Matzner, B. Marschner, and M. von Lützow, “An integrative approach of organic matter stabilization in temperate soils: linking chemistry, physics, and biology,” J. Plant Nutr. Soil Sci. 171 (1), 5–13 (2008). https://doi.org/10.1002/jpln.200700215
J. Kucerik, M. S. Demyan, and C. Siewert, “Practical application of thermogra-vimetry in soil science. Part 4. Relationship between clay, organic carbon and organic matter contents,” J. Therm. Anal. Calorim. 123, 2441–2450 (2016). https://doi.org/10.1007/s10973-015-5141-8
J. Kučerík, D. Tokarski, M. S. Demyan, I. Merbach, and C. Siewert, “Linking soil organic matter thermal stability with contents of clay, bound water, organic carbon and nitrogen,” Geoderma 316, 38–46 (2018). https://doi.org/10.1016/j.geoderma.2017.12.001
P. Leinweber, G. Jandl, C. Baum, K.-U. Eckhardt, and E. Kandeler, “Stability and composition of soil organic matter control respiration and soil enzyme activities,” Soil Biol. Biochem. 40 (6), 1496–1505 (2008). https://doi.org/10.1016/j.soilbio.2008.01.003
E. Lichtfouse, C. Chenu, F. Baudin, C. Leblond, M. da Silva, F. Béhar, S. Derenne, C. Largeau, P. Wehrung, and P. Albrecht, “A novel pathway of soil organic matter formation by selective preservation of resistant straight-chain biopolymers: chemical and isotope evidence,” Org. Geochem. 28 (6), 411–415 (1998). https://doi.org/10.1016/S0146-6380(98)00005-9
B. Marschner, S. Brodowski, A. Dreves, G. Gleixner, A. Gude, P. M. Grootes, U. Hamer, A. Heim, G. Jandl, R. Ji, K. Kaiser, K. Kalbitz, C. Kramer, P. Leinweber, J. Rethemeyer, et al., “How relevant is recalcitrance for the stabilization of organic matter in soils?” J. Plant Nutr. Soil Sci. 171, 91–110 (2008). https://doi.org/10.1002/jpln.200700049
A. Merino, A. Ferreiro, J. Salgado, M. T. Fonturbel, N. Barros, C. Fernández, and J. A. Vega, “Use of thermal analysis and solid-state 13C CP-MAS NMR spectroscopy to diagnose organic matter quality in relation to burn severity in Atlantic soils,” Geoderma 226–227, 376–386 (2014). https://doi.org/10.1016/j.geoderma.2014.03.009
F. Oudghiri, N. Allali, J. M. Quiroga, and M. R. Rodríguez-Barroso, “TG–FTIR analysis on pyrolysis and combustion of marine sediment,” Infrared Phys. Technol. 78, 268–274 (2016). https://doi.org/10.1016/j.infrared.2016.08.015
C. Peltre, J. M. Fernández, J. M. Craine, and A. F. Plante, “Relationships between biological and thermal indices of soil organic matter stability differ with soil organic carbon level,” Soil Sci. Soc. Am. J. 77 (6), 2020–2028 (2013). https://doi.org/10.2136/sssaj2013.02.0081
A. F. Plante, J. M. Fernández, and J. Leifeld, “Application of thermal analysis techniques in soil science,” Geoderma 153 (1–2), 1–10 (2009). https://doi.org/10.1016/j.geoderma.2009.08.016
A. F. Plante, J. M. Fernández, M. L. Haddix, J. M. Steinweg, and R. C. Conant, “Biological, chemical and thermal indices of soil organic matter stability in four grassland soils,” Soil Biol. Biochem. 43 (5), 1051–1058 (2011). https://doi.org/10.1016/j.soilbio.2011.01.024
J. Sanderman and A. S. Grandy, “Ramped thermal analysis for isolating biologically meaningful soil organic matter fractions with distinct residence times,” Soil 6, 131–144 (2020). https://doi.org/10.5194/soil-6-131-2020
M. Schiedung, A. Don, P. Wordell-Dietrich, V. Alcántara, P. Kuner, and G. Guggenberger, “Thermal oxidation does not fractionate soil organic carbon with differing biological stabilities,” J. Plant Nutr. Soil Sci. 180 (1), 18–26 (2017). https://doi.org/10.1002/jpln.201600172
H. R. Schulten and P. Leinweber, “Thermal stability and composition of mineral-bound organic matter in density fractions of soil,” Eur. J. Soil Sci. 50 (2), 237–248 (1999). https://doi.org/10.1046/j.1365-2389.1999.00241.x
C. Siewert, Investigation of the Thermal and Biological Stability of Soil Organic Matter (Shaker Verlag, Herzogenrath, 2001).
C. Siewert, M. S. Demyan, and J. Kučerík, “Interrelations between soil respiration and its thermal stability,” J. Therm. Anal. Calorim. 110 (1), 413–419 (2012). https://doi.org/10.1007/s10973-011-2099-z
P. Sollins, P. Homann, and B. A. Caldwell, “Stabilization and destabilization of soil organic matter: mechanisms and controls,” Geoderma 74, 65–105 (1996). https://doi.org/10.1016/S0016-7061(96)00036-5
D. Tokarski, J. Kučerík, K. Kalbitz, M. S. Demyan, I. Merbach, D. Barkusky, J. Ruehlmann, and C. Siewert, “Contribution of organic amendments to soil organic matter detected by thermogravimetry,” J. Plant Nutr. Soil Sci. 181 (5), 664–674 (2018). https://doi.org/10.1002/jpln.201700537
D. Tokarski, M. Wiesmeier, H. D. Weissmannová, K. Kalbitz, M. S. Demyan, J. Kučerík, and C. Siewert, “Linking thermogravimetric data with soil organic carbon fractions,” Geoderma 362, 114–124 (2020). https://doi.org/10.1016/j.geoderma.2019.114124
D. S. Volkov, O. B. Rogova, M. A. Proskurnin, Y. R. Farkhodov, and L. B. Markeeva, “Thermal stability of organic matter of typical chernozems under different land uses,” Soil Tillage Res. 197 (104500) (2020). https://doi.org/10.36291/HIT.2019.volkov.050
M. Lützow, I. Kögel-Knabner, K. Ekschmitt, H. Flessa, G. Guggenberger, E. Matzner, and B. Marschner, “SOM fractionation methods: relevance to functional pools and to stabilization mechanisms,” Soil Biol. Biochem. 39, 2183–2207 (2007). https://doi.org/10.1016/j.soilbio.2007.03.007
M. von Lützow, I. Kögel-Knabner, K. Ekschmitt, E. Matzner, G. Guggenberger, B. Marschner, and H. Flessa, “Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions—a review,” Eur. J. Soil Sci. 57, 426–445 (2006). https://doi.org/10.1111/j.1365-2389.2006.00809.x
Funding
The work was supported by the state budget (state task no. 0191-2019-0045) and by the Russian Science Foundation (project no. 17-14-01120p).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by G. Chirikova
The original online version of this article was revised due to a retrospective Open Access order.
Supplementary Information
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sokolov, D.A., Dmitrevskaya, I.I., Pautova, N.B. et al. A Study of Soil Organic Matter Stability Using Derivatography and Long-Term Incubation Methods. Eurasian Soil Sc. 54, 487–498 (2021). https://doi.org/10.1134/S1064229321040141
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1064229321040141