Abstract
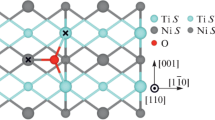
The atomic and electronic structure of the three surfaces of Ti3Al alloy—(0001), (\(1\bar 100\)), and (\(11\bar 20\))—is calculated by the projector augmented-wave method in the framework of the electron density functional theory. The surface energies are estimated as a function of the chemical potential of aluminum, which made it possible to construct a stability diagram for the surfaces under study. Adsorption of oxygen on differently oriented surfaces of the alloy is studied. It is found that the most preferred positions for oxygen adsorption are hollow positions on the (0001) and (\(11\bar 20\))Ti–Al surfaces and bridge positions on the (\(1\bar 100\))Ti‒Al-1 surface. Structural and electronic factors that determine these energy preferences are discussed. It is shown that regardless of the orientation of the surface, oxygen “prefers” titanium-enriched positions. The effect of oxygen on the atomic and electronic structure of low-index surfaces is discussed. It is found that at low concentrations of oxygen, the formation of its chemical bond with titanium and/or aluminum atoms in the surface and subsurface layers leads to the appearance of low-lying states split off from the bottom of the valence bands of metals, which is accompanied by the formation of a pseudogap and the weakening of Ti‒Al metal bonds in the surface layers.
Similar content being viewed by others
References
Z. Li and W. Gao, in Intermetallics Research Progress, Ed. by Y. N. Berdovsky (Nova Science, New York, 2008), p. 1.
I. Polmear, Light Alloys: From Traditional Alloys to Nanocrystals (Elsevier, Amsterdam, 2005, Tekhnosfera, Moscow, 2008).
F. H. Froes, C. Suryanarayana, and D. Eliezer, J. Mater. Sci. 27, 5113 (1992).
Y. Umakoshi, M. Yamaguchi, T. Sakagami, and T. Yamane, J. Mater. Sci. 24, 1599 (1989).
F. Dettenwanger and M. Schütze, Oxid. Met. 54, 121 (2000).
R. G. Reddy, JOM 54, 65 (2002).
M. P. Brady and P. F. Tortorelli, Intermetallics 12, 779 (2004).
J. G. Speight, Lange’s Handbook of Chemistry, 16th ed. (McGraw-Hill, New York, 2005), p. 124.
A. Y. Lozovoi, A. Alavi, and M. W. Finnis, Phys. Rev. Lett. 85, 610 (2000).
H. Li, S. Wang, and H. Ye, J. Mater. Sci. Technol. 25, 569 (2009).
S.-Y. Liu, J.-X. Shang, F.-H. Wang, and Y. Zhang, Phys. Rev. B 79, 075419 (2009).
L. Wang, J.-X. Shang, F.-H. Wang, Y. Zhang, and A. Chroneos, J. Phys.: Condens. Matter 23, 265009 (2011).
Y. Song, J. H. Dai, and R. Yang, Surf. Sci. 606, 852 (2012).
L. Wang, J.-X. Shang, F.-H. Wang, Y. Chen, and Y. Zhang, Acta Mater. 61, 1726 (2013).
S. E. Kulkova, A. V. Bakulin, Q. M. Hu, and R. Yang, Comput. Mater. Sci. 97, 55 (2015).
A. V. Bakulin, C. E. Kulkova, Q. M. Hu, and R. Yang, J. Exp. Theor. Phys. 120, 257 (2015).
A. M. Latyshev, A. V. Bakulin, S. E. Kulkova, Q. M. Hu, and R. Yang, J. Exp. Theor. Phys. 123, 991 (2016).
S.-Y. Liu, S. Liu, D. Li, T. M. Drwenski, W. Xue, H. Dang, and S. Wang, Phys. Chem. Chem. Phys. 14, 11160 (2012).
L.-J. Wei, J.-X. Guo, X.-H. Dai, Y.-L. Wang, and B.-T. Liu, Surf. Rev. Lett. 22, 1550053 (2015).
L.-J. Wei, J.-X. Guo, X.-H. Dai, L. Guan, Y.-L. Wang, and B.-T. Liu, Surf. Interface Anal. 48, 1337 (2016).
V. Maurice, G. Despert, S. Zanna, P. Josso, M.-P. Bacos, and P. Marcus, Acta Mater. 55, 3315 (2007).
P. E. Blöchl, Phys. Rev. B 50, 17953 (1994).
G. Kresse and J. Joubert, Phys. Rev. B 59, 1758 (1999).
G. Kresse and J. Hafner, Phys. Rev. B 48, 13115 (1993).
G. Kresse and J. Furthmüller, Phys. Rev. B 54, 11169 (1996).
G. Kresse and J. Furthmüller, Comp. Mater. Sci. 6, 15 (1996).
J. P. Perdew, K. Burke, and M. Ernzerhof, Phys. Rev. Lett. 77, 3865 (1996).
H. J. Monkhorst and J. D. Pack, Phys. Rev. B 13, 5188 (1976).
H. Shi and C. Stampfl, Phys. Rev. B 76, 075327 (2007).
K. P. Huber and G. Herzberg, Molecular Spectra and Molecular Structure IV: Constants of Diatomic Molecules (Van Nostrand Reinhold, New York, 1979).
Y. L. Liu, L. M. Liu, S. Q. Wang, and H. Q. Ye, Intermetallics 15, 428 (2007).
M. H. Yoo, J. Zou, and C. L. Fu, Mater. Sci. Eng. A 192–193, 14 (1995).
W. B. Pearson, A Handbook of Lattice Spacing and Structures of Metals and Alloys, 1st ed. (Pergamon, New York, 1958).
K. Tanaka, K. Okamoto, H. Inui, Y. Minonishi, M. Yamaguchi, and M. Koiwa, Philos. Mag. A 73, 1475 (1996).
F. D. Murnaghan, Proc. Natl. Acad. Sci. USA 30, 244 (1944).
T. Hong, T. J. Watson-Yang, X.-Q. Guo, A. J. Freeman, T. Oguchi, and J.-H. Xu, Phys. Rev. B 43, 1940 (1991).
D. Sornadurai, B. Panigrahi, and Ramani, J. Alloys Compd. 305, 35 (2000).
D. Music and J. M. Schneider, Phys. Rev. B 74, 174110 (2006).
Y. Wei, H.-B. Zhou, Y. Zhang, G.-H. Lu, and H. Xu, J. Phys.: Condens. Matter 23, 225504 (2011).
C. Y. Jones, W. E. Luecke, and E. Copland, Intermetallics 14, 54 (2006).
R. Hultgren, P. D. Desai, M. Gleiser, and D. T. Hawkins, Selected Values of Thermodynamic Properties of Binary Alloys (Am. Soc. Metals, Metals Park, OH, 1973).
F. R. de Boer, R. Boom, W. C. M. Mattens, A. R. Miedema, and A. K. Niessen, Cohesion in Metals: Transition Metal Alloys (North Holland, Amsterdam, 1989).
Smithells Metals References Book, Ed. by E. A. Brandes and G. B. Brook, 7th ed. (Butterworth-Heinemen, London, 1992).
L. Wang, J.-X. Shang, F.-H. Wang, and Y. Zhang, Appl. Surf. Sci. 276, 198 (2013).
G. Henkelman, B. P. Uberuaga, and H. Jónsson, J. Chem. Phys. 113, 9901 (2000).
T. I. Spiridonova, A. V. Bakulin, and S. E. Kul’kova, Phys. Solid State 57, 1921 (2015).
S. E. Kulkova, A. V. Bakulin, S. S. Kulkov, S. Hocker, and S. Schmauder, Phys. Scripta 90, 094010 (2015).
M. R. Shanabarger, Mater. Sci. Eng. A 153, 608 (1992).
M. R. Shanabarger, Appl. Surf. Sci. 134, 179 (1998).
J. Rüsing and C. Herzig, Intermetallics 4, 647 (1996).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.M. Latyshev, A.V. Bakulin, S.E. Kulkova, 2017, published in Fizika Tverdogo Tela, 2017, Vol. 59, No. 9, pp. 1828–1842.
Rights and permissions
About this article
Cite this article
Latyshev, A.M., Bakulin, A.V. & Kulkova, S.E. Adsorption of oxygen on low-index surfaces of Ti3Al alloy. Phys. Solid State 59, 1852–1866 (2017). https://doi.org/10.1134/S1063783417090165
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063783417090165