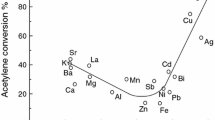
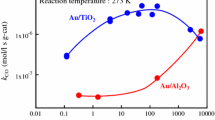
Abstract
The character of the catalytic oxidation of CO by supported gold cluster catalysts is analyzed with emphasis on the unique characteristics of this process. The scheme of this process used here has the reagent CO molecule captured in the interface between the cluster and support, with oxygen molecules or atoms located on the support surface to react with the CO. (Other models have also been presented.) The experimental data indicate that, together with configurational transitions that lead to the CO molecule joining an oxygen atom to form the CO2 molecule, the charge separation due to capture of the CO molecule by the supported gold cluster is important. The process of release of the CO2 molecule results in charge exchange; the time for this process is relatively long because of the large distance separating positive and negative charges, a distance exceeding the cluster radius. This provides a high efficiency of the oxidation of CO with this catalyst despite the relatively high activation energy for the configurational transition.
Similar content being viewed by others
References
R. Coquet, K. L. Howard, and J. Willock, Chem. Soc. Rev. 37, 2046 (2008).
F. Furche, R. Ahlrichs, P. Weis, C. Jacob, S. Gilb, T. Bierweiler, and M. M. Kappes, J. Chem. Phys. 117, 6982 (2002).
X. Xing, B. Yoon, U. Landman, and J. H. Parks, Phys. Rev. B: Condens. Matter 74, 165423 (2006).
J. Li, X. Li, H. J. Zhai, and L. S. Wang, Science (Washington) 299, 864 (2003).
H. Häkkinen, B. Yoon, U. Landman, X. Li, H.-J. Zhai, and L.-S. Wang, J. Phys. Chem. A 107, 6168 (2003).
X. Gu, M. Ji, S. H. Wei, and X. G. Gong, Phys. Rev. B: Condens. Matter 70, 205401 (2004).
W. Fa and J. Dong, J. Chem. Phys. 124, 114310 (2006).
H. Häkkinen, M. Moseler, O. Kostko, N. Morgner, M. A. Hoffmann, and B. V. Issendorff, Phys. Rev. Lett. 93, 093401 (2004).
J. Oviedo and R. E. Palmer, J. Chem. Phys. 117, 9548 (2002).
E. M. Fernández, J. M. Soler, I. L. Garzón, and L. C. Balbás, Phys. Rev. B: Condens. Matter 70, 165403 (2004).
H. Arslan and M. H. Güven, New J. Phys. 7, 60 (2005).
H. Arslan and M. H. Güven, Acta Phys. Slovaca 56, 511 (2006).
E. K. Yildirim, M. Atis, and Z. B. Guvenc, Phys. Scr. 75, 111 (2007).
M. Haruta, Catal. Today 36, 153 (1987).
M. Haruta, T. Kobayashi, H. Sato, and N. Yamada, Chem. Lett. 2, 405 (1987).
M. Haruta, N. Yamada, T. Kobayashi, and S. Iijima, J. Catal. 115, 301 (1989).
M. Haruta, Chem. Rec. 3, 75 (2003).
V. A. Bondzie, S. C. Parker and C. T. Campbell, Catal. Lett. 63, 143 (1999).
C. M. Chang and M. Y. Chou, Phys. Rev. Lett. 93, 133401 (2004).
A. A. Herzig, C. J. Kiely, A. F. Carley, P. Landon, and G. J. Hutchings, Science (Washington) 321, 1331 (2008).
A. Sanchez, S. Abbet, U. Heiz, W.-D. Schneider, H. Häkkinen, R. N. Barnett, and U. Landman, J. Phys. Chem. A 103, 9573 (1999).
B. K. Min, A. R. Alemozafar, D. Pinnaduwage, X. Deng, and C. M. Friend, J. Phys. Chem. B 110, 19833 (2006).
B. K. Min and C. M. Friend, Chem. Rev. 107, 2709 (2007).
D. J. Wales, J. P. K. Doye, M. A. Miller, P. N. Mortenson, and T. R. Walsh, Adv. Chem. Phys. 115, 1 (2000).
D. J. Wales, Energy Landscapes (Cambridge University Press, Cambridge, 2003).
B. M. Smirnov and R. S. Berry, Phase Transitions in Simple Atomic Systems (Springer, Heidelberg, 2007).
M. R. Hoare and P. Pal, Adv. Phys. 20, 161 (1971); M. R. Hoare and P. Pal, Adv. Phys. 24, 645 (1975).
M. R. Hoare, Adv. Chem. Phys. 40, 49 (1979).
F. H. Stillinger and T. A. Weber, Phys. Rev. A: At., Mol., Opt. Phys. 25, 978 (1982).
F. H. Stillinger and T. A. Weber, Phys. Rev. A: At., Mol., Opt. Phys. 28, 2408 (1983).
B. Vekhter, K. D. Ball, J. Rose, and R. S. Berry, J. Chem. Phys. 106, 4644 (1997).
R. S. Berry and B. M. Smirnov, Phys.-Usp. 52(2), 137 (2009).
J. Jellinek, T. L. Beck, and R. S. Berry, J. Chem. Phys. 84, 2783 (1986).
H. L. Davies, J. Jellinek, and R. S. Berry, J. Chem. Phys. 86, 6456 (1987).
R. S. Berry, T. L. Beck, H. L. Davis, and J. Jellinek, Adv. Chem. Phys. 90, 75 (1988).
H. P. Cheng and R. S. Berry, Phys. Rev. A: At., Mol., Opt. Phys. 45, 7969 (1992).
R. S. Berry, Chem. Rev. (Washington) 93, 2379 (1993).
R. S. Berry, in Theory of Atomic and Molecular Clusters, Ed. by J. Jellinek (Springer, Berlin, 1999), p. 1.
G. K. Boreskov, Heterogeneous Catalysis (Nova Science, New York, 2003).
G. Ertl, H. Knösinger, F. Schüth, and J. Weitkamp, Handbook of Heterogeneous Catalysis (Wiley, Weinheim, 2008).
G. Rothenberg, Catalysis: Concepts and Green Applications (Wiley, Weinheim, 2008).
K. Kolasinski, Surface Science: Foundations of Catalysis and Nanoscience (Wiley, Weinheim, 2008).
M. Haruta, Catal. Today. 36, 153 (1997).
M. Haruta, CATTECH 6(3), 102 (2002).
G. C. Bond, C. Louis, and D. T. Thompson, Catalysis by Gold (Imperial College Press, London, 2006).
H. Häkkinen, S. Abbet, A. Sanchez, U. Heiz, and U. Landman, Angew. Chem., Int. Ed. 42(11), 1297 (2003).
S. Arrhenius, Z. Phys. Chem. 28, 317 (1899).
G. C. Bond, Metal-Catalysed Reactions of Hydrocarbons (Springer, New York, 2005).
U. Heiz, T. M. Bernhardt, and U. Landman, in: Nanocatalysis, Ed. by U. Heiz and U. Landman (Wiley, Berlin, 2007).
C. Harding, V. Habilpour, S. Kunz, A. N.-S. Farnbacher, U. Heiz, B. Yoon, and U. Landman, J. Am. Chem. Soc. 131, 538 (2009).
M. Moseler, H. Häkkinen, and U. Landman, Phys. Rev. Lett. 89, 176103 (2002).
K. Koga, T. Ikeshoji, and K. Sugarawa, Phys. Rev. Lett. 92, 115507 (2004).
C. L. Cleveland, W. D. Luedtke, and U. Landman, Phys. Rev. Lett. 81, 2036 (1998).
Y. G. Chushak and L. S. Bartell, J. Phys. Chem. B 105, 11605 (2001).
H. S. Nam, N. M. Hwang, B. D. Yu, and J.-K. Yoon, Phys. Rev. Lett. 89, 275502 (2002).
R. S. Berry and B. M. Smirnov, J. Chem. Phys. 130, 064302 (2009).
H. S. W. Massey, Rep. Prog. Phys. 12, 248 (1949).
L. M. Molina and B. Hammer, Phys. Rev. B: Condens. Matter 69, 155424 (2004).
L. M. Molina and B. Hammer, Appl. Catal., A 291, 21 (2005).
R. Coquet, G. J. Hutchings, S. H. Taylor, and D. J. Willock, J. Mater. Chem. 16, 1978 (2006).
L. D. Landau and E. M. Lifshitz, Course of Theoretical Physics, Vol. 3: Quantum Mechanics: Non-Relativistic Theory (Nauka, Moscow, 1974; Pergamon, London, 1980).
C. W. Corti, R. J. Holliday, and D. V. Thompson, Phys. Rev. Lett. 95, 06102 (2005).
G. J. Hutchings, Gold Bull. (London) 37, 3 (2004).
C. H. Christensen and J. Nørskov, Science (Washington) 327, 278 (2010).
R. E. Kunz, P. Blaudeck, K. H. Hoffmann, and R. S. Berry, J. Chem. Phys. 108, 2576 (1998).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Berry, R.S., Smirnov, B.M. Charge separation in CO oxidation involving supported gold clusters. J. Exp. Theor. Phys. 113, 907–913 (2011). https://doi.org/10.1134/S1063776111140019
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063776111140019