Abstract
Many urgent problems of modern mineralogical crystallography, to which the papers of this thematic issue of the journal Kristallografiya (Crystallography Reports) are devoted, have been considered. It is shown how the use of advanced physicochemical methods enriches scientific concepts about the real structures of minerals and nature-like compounds, structural conditionality of their physical properties, forms of concentration of chemical elements in terrestrial shells, crystallogenesis conditions, structural transformations in deep geospheres, relationships between structure types, and their interpretations based on modern concepts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
It is well known that minerals are important in both industry and scientific research, aimed, in particular, at revealing the history of the evolution of planets, the Solar System, and the Universe as a whole. Currently, the knowledge about minerals cannot be developed without detailed crystallochemical information. It is provided by structural studies, which are carried out on crystals of increasingly complex compositions and constitutions. Along with accumulation of crystallochemical data, the purpose of these studies is to predict the behavior of crystalline phases under different physicochemical conditions.
The results of the fundamental research in the field of mineralogical crystallography are of key importance for not only the development of the concepts about natural crystalline materials. They are necessary for understanding the processes of formation and evolution of geological objects at all levels: minerals, rocks, and ores. Detailed knowledge of the structural principles of minerals with various compositions and structures is necessary for designing new functional materials with specified optical, magnetic, electric, mechanical, catalytic, sorption, electrochemical, and other properties. In addition, structural data on minerals allow to understand better the phenomena occurring in technosphere and ecosphere in the crystalline compounds entering the composition of most of building and structural materials and present in commercial wastes. Some of recent results related to the solution of the aforementioned problems are considered in the collection of articles Highlights in Mineralogical Crystallographу [1] and the review of this collection by Ferraris, professor of the Turin University [2].
Almost immediately after the discovery of X-ray diffraction (XRD) by M. Laue in 1912, it became obvious that XRD data concern not only geology but also materials science; in the second half of the XX century, their importance for biology became obvious. Hence, it is not surprising that crystallography is included in the courses of most of Russian universities as an important component of physics, materials science, chemistry, and biology (let alone its conventional relationship with mineralogy). It is sufficient to mention that, even in the pre-X-ray epoch of science development, the first out of four volumes of the fundamental treatise by Amedeo Carlo Avogadro Solid-State Physics was devoted entirely to crystallography (Fisica de’ corpi ponderabili, 4 volumi, Torino 1837–1841 (Physics of Ponderable Bodies (Matter), 4 vols.) (Fig. 1).
The 19th volume of the Notes in Mineralogy [3], which are regularly published by the European Mineralogy Union starting with 1994, is devoted to deep analysis of the modern approaches to understanding the real structures of minerals and the nature of the effects complicating them. The results of the use of most advanced physicochemical methods, which facilitate investigation of very small crystals (including nanoparticles), weakly crystallized samples, and disordered and aperiodic structures, as well as the solutions of some other scientific problems were reported in the articles of that volume. Undoubtedly, many of the results obtained will be developed in future crystallographic studies.
Traditionally, structural identification is an important part of mineralogical crystallography. The scientific concepts of the structural chemistry of minerals and main sections of their systematics have been enriched significantly due to the new instrumental possibilities and progress in software, thus facilitating further development of mineralogy and inorganic chemistry. As is well known, in the first structural refinements of halite and diamond, which were performed 85 years ago (in the “Bragg” epoch), crystals with sizes from 1 cm to several millimeters were applied. In the 1920s–1930s (epoch of V. Taylor and L. Pauling), crystals less than 1 mm in size could be analyzed due to the invention of hot-cathode tubes. Then, beginning with the 1960s, the progress in the software development and the use of more powerful X-ray systems made it possible to reduce the sizes of crystals studied to several hundredths of millimeter. Finally, since the 1970s, the possibilities of X-ray analysis have been expanded to a great extent due to the use of high-power synchrotron radiation, which makes it possible to investigate micrometer-sized rare minerals and understand better the evolution history and crystallization conditions of containing rocks.
In this context, it should be noted that powder XRD analysis has been experienced a kind of revival during the last decades; having become more than a simple “biometric image” of a specific mineral, it facilitates structural refinement, qualitative analysis of mixtures, and obtainment of structural information for samples under extreme conditions [3].
New correlations between the structures and physical properties of crystals are being revealed. The objects under study have also become much more complex. It should be reminded that, when studying the first structures (in particular, K+Al3+(SO4)2 ⋅ 12H2O alum), the boundary between the science and art was at a level of determining 11 independent parameters. Now, the number of refined parameters in modulated structures reaches several hundreds and is three orders of magnitude larger for the structures of biological crystals as compared with minerals. To date, the complexity of crystal structures is estimated not only based on the number of the refined parameters involved in refinement but also with application of the information-theory apparatus [4]. In the first approximation, this number increases with an increase in the number of atoms per unit cell and with lowering of its symmetry. For example, cation ordering in a unit cell is accompanied by a transition to a superstructure having a higher information density in comparison with the initial cell.
Along with the broadening of possibilities for studying crystal structures of minerals, new approaches in understanding their constitution, nature of their technologically important properties, and crystallochemical features of crystallogenesis have been developed in the last decades. In this context, one should mention the concept of anion-centered complexes, many statements of which were developed by the researchers from the worldwide-known crystallographic school of St. Petersburg University [5]. Initially, these conclusions were based on the detailed analysis of the crystal chemistry of a large group of minerals, related to fumarole exhalations of the Great Tolbachik Fissure Eruption, which occurred on the Kamchatka peninsula in 1974–1975. As a result, a fairly voluminous series of Cu-bearing minerals was selected, which contained additional O anions (that are not involved in traditionally selected tetrahedral complexes). Their tetrahedral coordination is formed by four Cu atoms; note that the Cu–O distances are shorter in comparison with the conventional distances in other structures. Later on, it was shown that [OCu4] tetrahedra can be combined into topologically different complexes. Based on these observations, Filatov et al. concluded that [OCu4] tetrahedra are the main conveyors of copper from the deep magmatic zone to the terrestrial surface. By now, anion-centered complexes have been found in minerals and inorganic compounds of different compositions. Their analysis has revealed structural conditionality of physical properties of many crystals.
The period from the beginning of the XX century is characterized by great progress in the development of scientific concepts about minerals. It is not a mere chance that Academician N.P. Yushkin (1936–2012) wrote the following: “The most informative indicator of mineralogy development is the number of known mineral forms at a certain historical moment.” Before 1800, there were less than 100 known independent mineral forms. After this historical milestone, the rate of discovering new minerals has been continuously increasing. For example, 87 minerals were discovered in the period from 1800 to 1819. Then, in the period from 1820 to 1919, 185 minerals on average were discovered each 20 years. From 1920 to 1939, 256 new minerals were described, and 342 minerals were added in the period from 1940 to 1959. Since 1960, 40–50 (and more than 100–150 in the last decade) new minerals were discovered annually. Thus, to date, the total number of minerals found on the Earth exceeds 5600 (Fig. 2). Obviously, this result is due to the fruitful cooperation of mineralogists and crystallographers.
An increase in the number of new minerals in the period from 1960 to 2015 [6].
The discovery of a new mineral is considered by the mineralogy community as a significant event. There are relatively few regions on the Earth where new minerals are more likely to be found. These are, primarily, Khibiny and Lovozero alkaline complexes on the Kola peninsula, where 219 minerals were discovered [7]. The fumarole systems of the Tolbachik volcano (Kamchatka) added 129 new minerals. The next in the list are the Långban deposit of iron–manganese ores (Värmland mining region, Sweden), where 75 minerals were found; Vesuvius potassium-rich lavas, skarn rocks, and fumaroles (Campania, Italy) with 66 new minerals; Tsumeb polymetallic deposit in Namibia with 73 new minerals; etc.
The characterization of new minerals is an important part of modern mineralogical crystallography. Sometimes even rare minerals indicate unusual conditions of their crystallization. One of recent examples of these conclusions is the analysis of the structural principles for 96 copper-containing minerals (oxysalts, chlorides, and oxides), related to volcanic fumaroles. As a result, the crystallochemical features of the minerals formed in hot zones of fumaroles (T > 473 K) and at moderate temperatures (T = 323–423 K) were determined. All high-temperature minerals do not contain hydrogen atoms, and Cu2+ cations are in the fourfold or fivefold coordination in them. In contrast, the second-group minerals contain generally OH anions and/or water molecules, whereas Cu2+ cations are prone to octahedral coordination [8].
Another example is the discovery of coesite (a rare silica polymorph), which facilitated the interpretation of high-pressure metamorphism and specific features of the petrology of surrounding rocks [9].
Pekov, a discoverer of more than 200 new minerals, has outlined a set of conditions favorable for finding previously unknown minerals. First, this is a geochemical peculiarity of mineral-forming medium, which leads to its enrichment with some rare elements. For example, at insignificant variations in the physicochemical conditions, boron can form compounds with similar compositions but significantly different structures.
However paradoxical it may sound, a deficit of particular conventional chemical elements also leads to the formation of unusual natural compounds. This condition facilitates concentration of rarer elements and crystallization of minerals with these elements in their composition. A deficit of silica leads to the formation of oxides of Al, Mg, Be, and other elements, which usually enter the composition of silicates.
Ultrahigh pressures in deep geospheres or a combination of a high temperature and atmospheric pressure, which is characteristic of the crystallization from fumarolic sublimates at the boundary between hot lavas and carbonate rocks near Earth’s surface, may also induce formation of minerals with unusual compositions or structures.
Living organisms can also be in involved in mineral-formation processes. These are primarily bacteria, whose activity leads to selective separation of some chemical elements. This effect is especially pronounced in the zones of surface oxidation of sulfide and arsenide ore deposits, where, in particular, malachite, azurite, and many copper arsenates with various structures are formed.
Mineral formation involves 72 out of 118 chemical elements. According to the data of Krivovichev and Charykova [10], the following elements are leaders in the number of mineral species (given in parentheses) formed with their participation: oxygen (4041), hydrogen (2755), silicon (1448), calcium (1139), sulfur (1025), aluminum (960), iron (917), sodium (914), copper (616), phosphorus (580), arsenic (575), and magnesium (550).
There is no doubt that the impressive rate of discovering minerals is related to the improvement of physicochemical methods in use (Fig. 3). The data in Fig. 3 indicate an increasing role of XRD, electron-probe, and spectroscopic studies at a significant decrease in the importance of wet chemistry in characterization of new minerals. In the 100-year period (from 1917 to 2016), 4046 forms (three quarters of the total number of minerals) were discovered.
Characteristic techniques used to describe new minerals: (1) XRD analysis, (2) electron-probe analysis, and (3) wet chemistry. Other curves are the data of thermogravimetry/differential thermal analysis (TG/DTA), IR spectroscopy, and Raman spectroscopy [11].
An analysis of these studies suggests that they are generally supported by state foundations. In the last 100 years, minerals were revealed in different countries; however, the number of the research groups involved in these studies did not exceed 20. Most of new minerals are discovered at universities and academic institutes (in total, 75%) and in museums (25%). Two thirds of new minerals were found in the samples obtained from mines and quarries or when developing mineral deposits [11]. Lunar samples and meteorites also produced some new minerals. There are many new minerals in peralkaline rocks and volcano-related fumaroles. The number of discoverers of new minerals rises constantly; it increased by a factor of 4 in 70 years and amounts currently to six.
In the XIX century, minerals were identified based on chemical analysis and study of crystal morphology. All data were generalized in reference books (e.g., Dana’s System of Mineralogy, Goldschmidt’s Atlas of Crystal Forms, etc.). The time when an experienced mineralogist could visually determine several tens of mineral forms was left far in the past due to the discovery of XRD analysis, which changed radically the approach to characterization of minerals.
Successful diagnostics of an unknown mineral is primarily related to the presence of a sufficient amount of standard reference data, containing the values of diffraction reflection intensities I and interplanar spacings d. A need for creating reference databases was understood immediately after the first XRD patterns were recorded. For example, an American researcher Hull, who analyzed an X-ray image of NaF in 1919, revealed that this compound, considered previously as chemically pure, contained an impurity of NaHF2 [12].
As Hull told, a NaF agent of chemically pure grade was delivered from stock and subjected to XRD analysis. Then the researchers synthesized NaF compound of very high purity and also recorded its XRD pattern. A comparison of both images (Fig. 4) showed that the first sample contained ~30–40% of impurities. To determine their compositions, XRD patterns of Na2CO3, NaCl, NaHF2, and other chemically similar compounds were obtained. Thus, it was found for the first time that a commercial sample contained specifically the NaHF2 impurity.
Three XRD patterns, which made it possible to reveal the NaHF2 impurity in chemically pure (c.p.) NaF agent [12].
The first XRD databases were created only in 1938, when an American researcher Hanawalt and his colleguages published a paper [13] devoted to the XRD identification of materials, which contained calculated powder XRD patterns for 100 compounds. Each powder XRD pattern started to be considered as a “fingerprint” of the corresponding chemical compound. Then, the American Society for Testing and Materials (ASTM) systematized the structural data. In 1941, all XRD spectra known by that time were published (with participation of ASTM) in the form of a file with a searching key proposed by Hanawalt. The key included the data on the three strongest reflections, chemical formula, and the number of the card with complete information about this compound. In the next years, the file was expanded to 2500 compounds. Thereafter, a committee on filling the file was organized under the patronage of ASTM, being then assisted by scientific organizations of the United Kingdom, France, and Canada. Soon the committee assumed the functions of an international organization and became completely independent in 1969. The database compiled by it started to be referred to as “Powder Diffraction File of the Joint Committee on Powder Diffraction Standards” (PDF JCPDS). In 1978, 14 international and national scientific communities (primarily, from the United States, Germany, Canada, Australia, France, the United Kingdom, and Japan) established the International Center for Diffraction Data (ICDD) under the patronage of the International Union of Crystallography. The ICDD database PDF-2 is assumed to be created in 1940. It consists of two independent parts, which include the data on inorganic and organic compounds. In 1985, the XRD data for all characterized compounds were computerized. The dynamics of filling the ICSD database with XRD data is shown in Fig. 5.
By August 2019, the Inorganic Crystal Structure Database (ICSD) of the University of Bonn included 426 000 XRD patterns of inorganic crystals, 75% of which contained structural data. Corundum numbers (I/Ic), necessary for determining the quantitative ratios of minerals in mixtures, are given for 325 900 structures. The data sample of minerals and their analogues contains 47 000 XRD patterns, with corundum numbers (I/Ic) reported for 36 000 structures. The database contains also 143 636 XRD patterns of metals and alloys.
It should be acknowledged that an attempt to determine a mineral based on only the powder XRD database, containing data on more than 5600 mineral forms, may be unsuccessful in the absence of any chemical information. Spectral data also help greatly to solve this problem, especially in gemology, where nondestructive experimental methods are highly important. That is why the popularity of the high-quality RRUFF database of IR and Raman spectra constantly increases (along with that of the powder XRD database, annually renewed by the ICDD) in the mineralogical practice. This base contains XRD and spectral data on 7000 minerals (including their varieties) and 3500 mineral forms. This concept is accepted for minerals characterized by structures of the same type and chemically similar compositions. The organization of RRUFF was sponsored by Michael Scott, who was the first chief executive officer of Apple Computer (in the period from February 1977 to March 1981). He is also known as a gemstone expert. In is of interest that the RRUFF project was named after the sound imitating Scott’s cat purring.
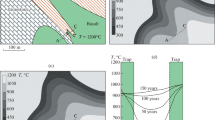
In the modern Earth sciences, the XRD analysis at high pressures is especially important, because the rocks located on the terrestrial surface and erupted from depths of less than 100 km make it possible to estimate the composition of only 0.02% of the Earth’s volume. In the last 50 years, the scientific concepts about the mineralogy of deep geospheres have been gradually expanded [14]. The results of these interdisciplinary studies are shown in a generalized form in Fig. 6.
From the point of view of mantle mineralogy, diamond inclusions are of particular interest, because they characterize the deep medium in which these high-pressure crystals grow. The recently found inclusion of OH-containing ringwoodite Mg2SiO4 in diamond (Fig. 7) (one of possible mantle components at depths in the range of 520–670 km [16]) indicates that diamond can be formed not only in the mantle but also in the transition zone with participation of hydrogen-containing melts or fluids. It is assumed that there are vacancies of Mg2+ cations in OH-containing ringwoodite, which occupy the only site in oxygen octahedra in this high-symmetry structure, whose sites can be occupied by the protons involved in the formation of OH groups. This isomorphism is quite possible in the wadsleyite structure; however, its lower-symmetry structure contains only one out of four crystallographically nonequivalent Mg octahedra, into which protons can be incorporated; therefore, its ability to accumulate water in the lower half of the mantle transition zone should be lower than that of ringwoodite. Nevertheless, both minerals (ringwoodite and wadsleyite) can be considered as important accumulators of water in deep geospheres [17].
(a) Ringwoodite inclusion in the diamond crystal and (b) map of Brazil with indication of the Juina kimberlite pipe, where the diamond with ringwoodite inclusion was found [15].
Another discovery is related to the identified inclusions of metallic iron and cohenite Fe3C in diamond [18]. It suggested that the diamond crystals containing these inclusions could be formed at much larger depths (~700 km) in comparison with the previously investigated samples of this mineral. At the same time, the modern potential of XRD experiments in chambers with diamond anvils allows one to predict possible mineralogical phases in not only the Earth’s mantle but also in the core of our planet.
New data indicate the existence of structural differences between the surface layers of different faces. The need for achieving electrostatic balance of dangling bonds leads to the fact that the structures of the crystal surface and crystal bulk have different geometries, symmetries, and properties. Some surprising examples (see below) confirm this conclusion. It is well known that diamond is a typical insulator (due to the two-electron covalent carbon interatomic bonds, which determine the wide band gap ΔE = 5.5 eV), whereas its surface with unsaturated bonds of carbon atoms, doped with hydrogen atoms, exhibits pronounced semiconductor properties [19]. A change in the electronic structure of pyrite, caused by shortened Fe–S bonds on its surface, is accompanied by the manifestation of nonmetallic metalloid properties (with covalent type of interatomic interactions) at the crystal surface, in contrast to the semiconductor conductivity of the crystal as a whole. It may seem surprising that magnetite, which is a semimetal with covalent bonds, has ferromagnetic properties; however, their nature is due to the exchange interaction between iron atoms.
Recently, according to the opinion of some researchers, some major discoveries were made in a relatively new line of research, related to quasicrystals. The formation and characterization of first quasicrystals in 1984 were preceded by the description of incommensurate periodicity. Thus, it was found that the periodicity of three-dimensional atomic distribution in real space is not a necessary property of crystals (in contrast to the conservation of periodicity in the reciprocal (diffraction) space). The relatively rare manifestation of quasicrystallinity among synthetic intermetallic compounds with nonclassical symmetries suggests that many quasicrystals are metastable phases. However, the recent finding of three natural quasicrystals in the meteorites from Chukotka (the first quasicrystalline mineral icosahedrite (Al63Cu24Fe13) was discovered in 2009) and previously investigated synthetic intermetallic compounds left no doubt that this form of matter is stable and important for the Earth sciences [20]. In addition to mineralogy, these data undoubtedly offer new prospects in many natural sciences: astrophysics (by expanding the ideas about possible processes in the early development stages of the Solar System), solid-state physics, and materials science. It remains to be said that, from the point of view of data capacity, quasicrystals can be considered as a bridge from minerals to, on the one hand, inorganic materials and, on the other hand, biocrystals.
A relationship between crystallography and modern biology and medicine was noted above. Currently, no medical product can be recommended without crystallographic analysis of its structure. It can also be assumed that argillaceous minerals and zeolites played a certain role in the origin of life on the Earth [21]. Some aspects of this extremely complex and intriguing problem were considered in the study Generated from Crystals? by Academician Yushkin [22]. He noted a surprising similarity between the simplest biosystems and hydrocarbon crystals with similar structures. One can obtain a real protoorganism by supplementing this mineral with protein components. Many geological aspects of the abiotic synthesis of organic materials and multimolecular systems using gradual transformations of inanimate nature were considered by Rutten [23] (Fig. 8).
Along with the transmission electron microscopy (TEM), a relatively new method of automatic XRD tomography is being gradually introduced into the mineralogical practice. It is especially efficient for studying nanocrystals, including biominerals in living organisms (including humans). New data were obtained on vaterite, which is a polymorphic form of calcite present in the shells of gastropods; on hydroxylapatite in human dental enamel; and on silicon-containing nanorods of primary sponges. Currently, different forms of proteins are under study.
In the aforementioned vaterite structure, planes of CO3 triangles are almost parallel to the principal axis of the structure. Triangular CO3 anions in the structures of bastnäsite-(Ce) CeCO3F, parisite-(Ce) Ce2CaF2(CO3)3, and synchysite-(Ce) CaREE(CO3)2F (REE is a rare-earth elements) have a similar vertical orientation. Along with vaterite, these structures form a unified polysomatic family [24]; its representatives illustrate the applicability of the modular concept for their interpretation, due to which the relationships between individual structure types can be understood better.
The results considered above only partially characterize the range of problems crystallographers and mineralogists currently deal with. In this context, it should be reminded that crystallography originated in the XVIII century at the interface of mineralogy and mathematics and continues to develop in many respects specifically due to the study of minerals. It is not surprising in this context that, at the initiative of professor G. Ferraris (Italy) and the author of this study, a subdivision named as Mineralogical Crystallography, focused on modern high-tech structural studies of minerals and nature-like compounds, was established within the European Crystallographic Association at the IUCr congress in Geneva in 2002. This fact is an additional confirmation of the postulate that mineralogical crystallography is not static but actively developing line of research, the results of which expand the concepts in different fields of natural science.
Change history
19 April 2022
An Erratum to this paper has been published: https://doi.org/10.1134/S1063774521340071
REFERENCES
Highlights in Mineralogical Crystallography, Ed. by Th. Armbruster and R. M. Danisi (De Gruyter, Berlin, 2015).
G. Ferraris, Crystallogr. Rev. 22 (4), 280 (2016). https://doi.org/10.1080/0889311x.2016.1157788
Mineralogical Crystallography, Ed. by J. Plášil, J. Majzlan, and S. Krivovichev, EMU Notes in Mineralogy (European Mineralogical Union and Mineralogical Society of Great Britain and Ireland, London, 2017), Vol. 19.
S. Krivovichev, Acta Crystallogr. A 68, 393 (2012).
S. V. Krivovichev, O. Mentré, O. I. Siidra, et al., Chem. Rev. 113 (8), 6459 (2013). https://doi.org/10.1021/cr3004696
E. S. Grew, G. Hystad, R. M. Hazen, et al., Am. Mineral. 102 (8), 1573 (2017). https://doi.org/10.2138/am-2017-5897
I. V. Pekov, Soros Obrazovat. Zh., No. 5, 65 (2001).
I. V. Pekov, N. V. Zubkova, and D. Yu. Pushcharovsky, Acta Crystallogr. B 74, 502 (2018). https://doi.org/10.1107/S2052520618014403
C. Chopin, Contrib. Mineral. Petrol. 86, 107 (1984).
V. G. Krivovichev and M. V. Charykova, Zap. Ross. Mineral. O-va, No. 4, 1 (2015).
I. F. Barton, Am. Mineral. 104 (5), 641 (2019).
A. W. Hull, J. Am. Chem. Soc. 41 (8), 1168 (1919). https://doi.org/10.1021/ja02229a003
J. D. Hanawalt, H. W. Rinn, and L. K. Frevel, Ind. Eng. Chem. Anal. Ed. 10 (9), 457 (1938).
D. Pushcharovsky and Yu. Pushcharovsky, Earth-Sci. Rev. 113 (2), 94 (2012).
F. Nestola and J. R. Smyth, Int. Geol. Rev. 58 (3), 263 (2015). https://doi.org/10.1080/00206814.2015.1056758
D. G. Pearson, F. E. Brenker, F. Nestola, et al., Nature 507, 221 (2014). https://doi.org/10.1038/nature13080
N. Purevjav, T. Okuchi, X. Wang, et al., Acta Crystallogr. B 74 (1), 115 (2018). https://doi.org/10.1107/s2052520618000616
E. M. Smith, S. B. Shirey, F. Nestola, et al., Science 354 (6318), 1403 (2016). https://doi.org/10.1126/science.aal1303
J. Ristein, Appl. Phys. A 82 (3), 377 (2005). https://doi.org/10.1007/s00339-005-3363-5
L. Bindi, P. J. Steinhardt, N. Yao, and P. J. Lu, Science 324 (5932), 1306 (2009). https://doi.org/10.1126/science.1170827
J. D. Bernal, The Origin of Life (Weidenfeld and Nicolson, London, 1967).
N. P. Yushkin, Nauka iz Pervykh Ruk, No. 1, 42 (2004).
M. Rutten, Origin of Life by Natural Causes (Elsevier, New York, 1971).
G. Capitani, Minerals 10 (1), 77 (2020). https://doi.org/10.3390/min10010077
Funding
This study was supported by the Russian Foundation for Basic Research, project no. 18-05-00332.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by Yu. Sin’kov
The original online version of this article was revised due to a retrospective Open Access order.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pushcharovsky, D.Y. Mineralogical Crystallography: Look in the Past, New Trends, and Highlights. Crystallogr. Rep. 66, 2–9 (2021). https://doi.org/10.1134/S1063774521010132
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063774521010132