Abstract
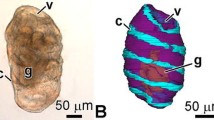
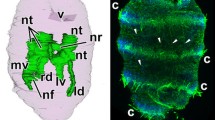
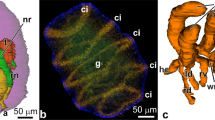
The differentiation of the ectodermal, entodermal, and mesodermal cell lines in developing plutei of the ophiuroid Amphipholis kochii was examined using electron microscopy and the immunochemical staining technique. The ectodermal cells form the pseudostratified epithelium of the ciliary band, the flattened epithelium of the body wall, and the esophageal epithelium. The epithelium of the ciliary band consists of ciliated and mucous cells; at its base is an axonal tract formed of the processes of neurons. The serotoninergic neurons form two lateral ganglia located along the paraoral ciliary band and the posterolateral arms’ ciliary band. The prominent features of the neurons are large size, the presence of a cilium, an electron-light cytoplasm filled with microvesicles with neurotransmitters, and a large nucleus with a predominant euchromatin. The ectoderm cells (except mucous cells) are characterized by the presence of a cilium surrounded by a collar of microvilli and a thin layer of apical extracellular matrix. The entodermal cells form the digestive tract epithelium and differentiate into four cell types: type I and II cells probably function in the nutrient uptake and assimilation; type III cells perhaps secrete digestive enzymes; and myoepithelial cells that constitute the cardiac and pyloric sphincters and the anus. Sclerenchymatous cells, which are the descendants of the primary mesenchyme, form a syncytium around the developing spicules. The biomineralization process is intrasyncytial, the ophioplutei spicules retain the cytoplasmic covering throughout the period of larval development. The secondary mesenchyme gives rise to smooth muscle cells and amebocytes. Muscle cells compose the circumesophageal musculature, the cell processes of each “muscle band” seem to fuse together. At the base of the preoral band are two symmetrically located groups of muscles, viz., the anterior dilators. Amebocytes function in excretion either near the epidermis or are able to penetrate through the epidermis and excrete wastes into the external environment. The mesoderm formed by the enterocoely gives rise to three pairs of coeloms; their cells remain unspecialized during the entire period of larval development. Results of this study are compared with the micro- and neuroanatomy of the larvae of other echinoderms.
Similar content being viewed by others
References
Gliznutsa, L.A. and Dautov, S.Sh., Ultrastructural Peculiarities of the Embryogenesis of the Brittle Star Amphipholis kochii (Lütken, 1872), Russ. J. Mar. Biol., 2005, vol. 31, no. 3, pp. 194–201.
Ivanova-Kazas, O.M., Evolyutsionnaya embriologiya zhivotnykh (Evolutionary Embryology of Animals), Saint Petersburg: Nauka, 1995.
Isaeva, V.V., Kletki v morfogeneze (Cells in Morphogenesis), Moscow: Nauka, 1994.
Kashenko, S.D., Effect of Desalination on Development of Far Eastern Trepang, Russ. J. Mar. Biol., 1992, vol. 18, nos. 3–4, pp. 43–52.
Kryuchkova, G.A., Development of the Brittle Stars Ophiura sarsi and Amphipholis kochii, Biol. Morya, 1988, no. 1, pp. 33–40.
Filimonova, G.F., Funktsional’naya morfologiya pishchevaritel’noi sistemy iglokozhikh (Functional Morphology of the Digestive System of Echinoderms), Leningrad: Nauka, 1979.
Yushin, V.V. and Nezlin, L.P., Ultrastructure of the Ciliary Band of the Echinopluteus—A Planktonic Larva of the Sea Urchin Echinocardium cordatum, Tsitologiya, 1990, vol. 32, no. 5, pp. 469–476.
Yushin, V.V., Bukhartseva, N.V., and Malakhov, V.V., Electron Microscopic Study of Development of the Far Eastern Sea Cucumber Stichopus japonicus from Blastula to Dipleurula, Tsitologiya, 1993, vol. 35, no. 1. pp. 22–29.
Abed, M. and Crawford, B.J., Ultrastructural Aspects of Mouth Formation in the Starfish Pisaster ochraceus, J. Morphol., 1986, vol. 188, pp. 239–250.
Amey, L., Compere, Ph., Dille, J., and Dubois, Ph., Ultrastructure and Cytochemistry of the Early Calcification Site and of Its Mineralization Organic Matrix in Paracentrotus lividus (Echinodermata: Echinoidea), Histochem. Cell Biol., 1998, vol. 110, pp. 285–294.
Beer, A.-J., Moss, C., and Thorndyke, M., Development of Serotonin-like and SALMFamide-like Immunoreactivity in the Nervous System of the Sea Urchin Psammechinus miliaris, Biol. Bull., 2001, vol. 200, pp. 268–280.
Burke, R.D. and Alvarez, C., Development of the Esophageal Muscles in Embryos of the Sea Urchin Strongylocentrotus purpuratus, Cell Tissue Res., 1988, vol. 252, pp. 411–417.
Byrne, M., Ophiuroidea, Microscopic Anatomy of Invertebrates, Harrison, F.W. and Chia, F.S., Eds., New York: Wiley-Liss, 1994, vol. 14, pp. 247–344.
Byrne, M. and Cisternas, P., Development and Distribution of the Peptidergic System in Larval and Adult Patiriella: Comparison of Sea Star Bilateral and Radial Nervous Systems, J. Comp. Neurol., 2002, vol. 451, pp. 101–114.
Byrne, M., Emlet, R., and Cerra, A., Ciliated Band Structure in Planktotrophic and Lecithotrophic Larvae of Heliocidaris Species (Echinodermata: Echinoidea): A Demonstration of Conservation and Change, Acta Zool., 2001, vol. 82, pp. 189–199.
Byrne, M., Nakajima, Y., Chee, F., and Burke, R., Apical Organs in Echinoderm Larvae: Insights into Larval Evolution in the Ambulacraria, Evol. Dev., 2007, vol. 9, no. 5, pp. 432–445.
Byrne, M., Sewell, M., Selvakumaraswamy, P., and Prowset, T., The Larval Apical Organ in the Holothuroid Chiridota gigas (Apodida): Inferences on Evolution of the Ambulacrarian Larval Nervous System, Biol. Bull., 2006, vol. 211, pp. 95–100.
Cameron, A. and Holland, N., Electron Microscopy of Extracellular Materials During the Development of a Sea Star, Patiria miniata (Echinodermata: Asteroidea), Cell Tissue Res., 1983, vol. 234, pp. 193–200.
Chee, F. and Byrne, M., Development of the Larval Serotonergic Nervous System in the Sea Star Patiriella regularis as Revealed by Confocal Imaging, Biol. Bull., 1999, vol. 197, pp. 123–131.
Chia, F.-S., Scanning Electron Microscopic Observation of the Mesenchyme Cells in the Larvae of the Starfish Pisaster ochraceus, Acta Zool., 1977, vol. 58, pp. 45–51.
Chia, F.-S. and Burke, R.D., Echinoderm Metamorphosis: Fate of Larval Structures, Settlement and Metamorphosis of Marine Invertebrate Larvae, New York: Elsevier, 1978, pp. 219–246.
Chia, F.-S. and Xing, J., Echinoderm Coelomocytes, Zool. Stud., 1996, vol. 35, no. 4, pp. 231–254.
Cisternas, P. and Byrne, M., Peptidergic and Serotonergic Immunoreactivity in the Metamorphosing Ophiopluteus of Ophiactis resiliens (Echinodermata, Ophiuroidea), Invertebr. Biol., 2003, vol. 122, no. 2, pp. 177–185.
Dupont, S., Thorndyke, W., Thorndyke, W., and Burke, R., Neural Development of the Brittlestar Amphiura filiformis, Dev. Genes Evol., 2009, vol. 219, pp. 159–166.
Hart, M., Life History Evolution and Comparative Developmental Biology of Echinoderms, Evol. Dev., 2002, vol. 4, no. 1, pp. 62–71.
Hirokawa, T., Komatsu, M., and Nakajima, Y., Development of the Nervous System in the Brittle Star Amphipholis kochii, Dev. Genes Evol., 2008, vol. 218, pp. 15–21.
Jangoux, M., Digestive Systems: Ophiuroidea, Echinoderm Nutrition, Rotterdam: A.A. Balkema Publishers, 1982, pp. 273–279.
Janies, D., Phylogenetic Relationships of Extant Echinoderm Classes, Can. J. Zool., 2001, vol. 79, no. 7, pp. 1232–1250.
Kungurtzeva, L. and Dautov, S., Ultrastructure of Digestive Tract in the Ophiopluteus of Ophiura sarsi, Invert. Reprod. Dev., 2001, vol. 39, no. 3, pp. 209–220.
Lacalli, Th., Mesodermal Pattern and Pattern Repeats in the Starfish Bipinnaria Larva, and Related Patterns in Other Deuterostome Larvae and Chordates, Phil. Trans. Roy. Soc. London, 1996, vol. 351, pp. 1737–1758.
Lacalli, Th.C. and Kelly, S.J., Anterior Neural Centers in Echinoderm Bipinnaria and Auricularia Larvae: Cell Types and Organization, Acta Zool., 2002, vol. 83, pp. 99–110.
Lacalli, Th., Gilmour, T., and West, J., Ciliary Band Innervation in the Bipinnaria Larva of Pisaster ochraceus, Phil. Trans. Roy. Soc. London, 1990, vol. 330, pp. 371–390.
Nakajima, Y., Presence of a Ciliary Patch in Preoral Epithelium of Sea Urchin Plutei, Develop. Growth Differ., 1986, vol. 28, no. 3, pp. 243–249.
Nakajima, Y., Burke, R., and Noda, Y., The Structure and Development of the Apical Ganglion in the Sea Urchin Pluteus Larvae of Strongylocentrotus droebachiensis and Mespilia globules, Develop. Growth Differ., 1993, vol. 35, no. 5, pp. 531–538.
Nakajima, Y., Kaneko, H., Murray, G., and Burke, R.D., Divergent Patterns of Neural Development in Larval Echinoids and Asteroids, Evol. Dev., 2004, vol. 6, no. 2, pp. 95–104.
Nezlin, L. and Yushin, V., The Digestive Tract of the Echinopluteus of Echinocardium cordatum (Echinodermata, Echinoida): Its Ultrastructure and Innervation, Can. J. Zool., 1994, vol. 72, pp. 2090–2099.
Ryberg, E., Development and Possible Function of the Red Pigment in Sea Urchin Larvae, Proc. of the European Colloquium on Echinoderms, Brussels, 3–8 September 1979, Rotterdam: A.A. Balkema, 1980, pp. 405–407.
Ryberg, E. and Lundgren, B., Some Aspects of Pigment Cells Distribution and Function in the Developing Echinopluteus of Psammechinus miliaris, Develop. Growth Differ., 1979, vol. 21, no. 2, pp. 129–140.
Sato, Y., Kaneko, H., Negishi, S., and Yazaki, I., Larval Arm Resorption Proceeds Concomitantly with Programmed Cell Death During Metamorphosis of the Sea Urchin Hemicentrotus pulcherrimus, Cell Tissue Res., 2006, vol. 326, pp. 851–860.
Spiegel, E. and Howard, L., Development of Cell Junctions in Sea-Urchin Embryos, J. Cell Sci., 1983, vol. 62, pp. 27–48.
Strathmann, R., Larval Feeding in Echinoderms, Am. Zool., 1975, vol. 1, pp. 717–730.
Takata, H. and Kominami, T., Behavior and Differentiation Process of Pigment Cells in a Tropical Sea Urchin Echinometra mathaei, Develop. Growth Differ., 2003, no. 45, pp. 473–483.
Tokuoka, M., Setoguchi, C., and Kominami, T., Specification and Differentiation Processes of Secondary Mesenchyme-derived Cells in Embryos of the Sea Urchin Hemicentrotus pulcherrimus, Develop. Growth Differ., 2002, vol. 44, no. 3, pp. 239–250.
Yaguchi, Sh., Yaguchi, J., and Burke, R., Specification of Ectoderm Restricts the Size of the Animal Plate and Patterns Neurogenesis in Sea Urchin Embryos, Development, 2006, vol. 133, pp. 2337–2346.
Yamashita, M., Embryonic Development of the Brittle-Star Amphipholis kochii in Laboratory Culture, Biol. Bull., 1985, vol. 169, pp. 131–142.
Yokota, Y. and Sappington, T.W., Vitellogen and Vitellogenin in Echinoderm, Reproductive Biology of Invertebrates, Raikhel, A.S. and Sappington, T.W., Eds., vol.12, part A, 2001, pp. 200–221.
Yokota, Y., Kato, K.H. and Mita, M., Morphological and Biochemical Studies on Yolk Degradation in the Sea Urchin Hemicentrotus pulcherrimus, Zool. Sci., 1993, vol. 10, pp. 661–670.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © L.A. Gliznutsa, S.Sh. Dautov, 2011, published in Biologiya Morya.
Rights and permissions
About this article
Cite this article
Gliznutsa, L.A., Dautov, S.S. Cell differentiation during the larval development of the ophiuroid Amphipholis kochii Lütken, 1872 (Echinodermata: Ophiuroidea). Russ J Mar Biol 37, 384–400 (2011). https://doi.org/10.1134/S1063074011050051
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063074011050051