Abstract
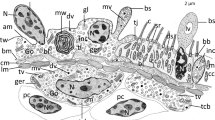
Sea urchins are excellent models to elucidate metamorphic phenomena of echinoderms. However, little attention has been paid to the way that their organ resorption is accomplished by programmed cell death (PCD) and related cellular processes. We have used cytohistochemistry and transmission electron microscopy to study arm resorption in competent larvae of metamorphosing sea urchins, Hemicentrotus pulcherrimus, induced to metamorphose by L-glutamine treatment. The results show that: (1) columnar epithelial cells, which are constituents of the ciliary band, undergo PCD in an overlapping fashion with apoptosis and autophagic cell death; (2) squamous epithelial cells, which are distributed between the two arrays of the ciliary band, display a type of PCD distinct from that of columnar epithelial cells, i.e., a cytoplasmic type of non-lysosomal vacuolated cell death; (3) epithelial integrity is preserved even when PCD occurs in constituent cells of the epithelium; (4) secondary mesenchyme cells, probably blastocoelar cells, contribute to the elimination of dying epithelial cells; (5) nerve cells have a delayed initiation of PCD. Taken together, our data indicate that arm resorption in sea urchins proceeds concomitantly with various types of PCD followed by heterophagic elimination, but that epithelial organization is preserved during metamorphosis.









Similar content being viewed by others
References
Araujo H, Machado LCH, Octacílio-Silva S, Mizutani CM, Silva MJF, Ramos RGP (2003) Requirement of the roughest gene for differentiation and time of death of interommatidial cells during pupal stages of Drosophila compound eye development. Mech Dev 120:537–547
Beer A-J, Moss C, Thorndyke M (2001) Development of serotonin-like and SALMFamide-like immunoreactivity in the nervous system of the sea urchin Psammechinus miliaris. Biol Bull 200:268–280
Berry DL, Schwartzman RA, Brown DD (1998) The expression pattern of thyroid hormone response genes in the tadpole tail identifies multiple resorption programs. Dev Biol 203:12–23
Biederbick A, Kern HF, Elsässer HP (1995) Monodansylcadaverine (MDC) is a specific in vivo marker for autophagic vacuoles. Eur J Cell Biol 66:3–14
Bowen ID, Morgan SM, Mullarkey K (1993) Cell death in the salivary glands of metamorphosing Calliphora vomitoria. Cell Biol Int 17:13–33
Burke RD (1983) Neural control of metamorphosis in Dendraster excentricus. Biol Bull 164:176–188
Cameron RA, Fraser S, Britten RJ, Davidson EH (1991) Macromere cell fates during sea urchin development. Development 113:1085–1091
Chia F-S, Burke RD (1978) Echinoderm metamorphosis: fate of larval structures. In: Chia F-S, Rice ME (eds) Settlement and metamorphosis of marine invertebrate larvae. Elsevier, New York, pp 219–234
Clarke PGH (1990) Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol 181:195–213
Dai J-D, Gilbert LI (1997) Programmed cell death of the prothoracic glands of Manduca sexta during pupal-adult metamorphosis. Insect Biochem Mol Biol 27:69–78
Dan-Sohkawa M, Suzuki J, Towa S, Kaneko H (1993) A comparative study on the fusogenic nature of echinoderm and nonechinoderm phagocytes in vitro. J Exp Zool 267:67–75
Das B, Schreiber AM, Huang H, Brown DD (2002) Multiple thyroid hormone-induced muscle growth and death programs during metamorphosis in Xenopus laevis. Proc Natl Acad Sci USA 99:12230–12235
Elinson RP, Remo B, Brown DD (1999) Novel structural elements identified during tail resorption in Xenopus laevis metamorphosis: lessons from tailed frogs. Dev Biol 215:243–252
Estabel J, Mercer A, Köning N, Exbrayat J-M (2003) Programmed cell death in Xenopus laevis spinal cord, tail and other tissues, prior to, and during, metamorphosis. Life Sci 73:3297–3306
Fox H (1973) Degeneration of the nerve cord in the tail of Rana temporaria during metamorphic climax: study by electron microscopy. J Embryol Exp Morphol 30:377–396
Hiesinger PR, Reiter C, Schau H, Fischbach K-F (1999) Neuropil pattern formation and regulation of cell adhesion molecules in Drosophila optic lobe development depend on synaptobrevin. J Neurosci 19:7548–7556
Ishizuya-Oka A (1996) Apoptosis of larval cells during amphibian metamorphosis. Microsc Res Tech 34:228–235
Kinch G, Hoffman KL, Rodrigues EM, Zee MC, Weeks JC (2003) Steroid-triggered programmed cell death of a motoneuron is autophagic and involves structural changes in mitochondria. J Comp Neurol 457:384–403
Menon J, Gardner EE, Vail S (2000) Developmental implications of differential effects of calcium in tail and body skin of Anuran tadpoles. J Morphol 244:31–43
Metchnikoff E (1968) Lecture V. In: Lectures on the comparative pathology of inflammation. Dover, New York, pp 56–74
Nakajima Y, Kaneko H, Murray G, Burke RD (2004) Divergent patterns of neural development in larval echinoids and asteroids. Evol Dev 6:95–104
Papadimitriou JM, Walters MN (1979) Macrophage polykarya. CRC Crit Rev Toxicol 6:211–255
Pavans de Ceccatty M (1982) In vitro aggregation of syncytia and cells of a Hexactinellida sponge. Dev Comp Immunol 6:15–22
Sato Y (2003) Apoptosis of retracting larval tissues during metamorphosis of sea urchin. Hiyoshi Rev Nat Sci Keio University 35:19–27 (in Japanese)
Sato Y, Yazaki I (1999) A cellular analysis of sea urcin metamorphosis induced by L-glutamine. In: Carnevali MDC, Bonasoro F (eds) Echinoderm research 1998. Balkema, Rotterdam, pp 221–226
Schweichel JU, Merker HJ (1973) The morphology of various types of cell death in prenatal tissues. Teratology 7:253–266
Seipp S, Schmich J, Leitz T (2001) Apoptosis—a death-inducing mechanism tightly linked with morphogenesis in Hydractina echinata (Cnidaria, Hydrozoa). Development 128:4891–4898
Silva JRMC (2000) The onset of phagocytosis and identity in the embryo of Lytechinus variegatus. Dev Comp Immunol 24:733–739
Silva JRMCD (2002) Role of the phagocytes on embryos: some morphological aspects. Microsc Res Tech 57:498–506
Tamboline CR, Burke RD (1992) Secondary mesenchyme of the sea urchin embryo: ontogeny of blastocoelar cells. J Exp Zool 262:51–60
Tamura M, Dan-Sohkawa M, Kaneko H (1998) Coelomic pouch formation in reconstructing embryos of the starfish Asterina pectinifera. Dev Growth Differ 40:567–575
Taniguchi K, Kurata K, Maruzoi T, Suzuki M (1994) Dibromomethane, a chemical inducer of larval settlement and metamorphosis of the sea urchin Strongylocentrotus nudus. Fisheries Sci 60:795–796
Yazaki I (1995) Quantitative analysis of metamorphosis induced by L-glutamine in embryos of the sea urchin, Hemicentrotus pulcherrimus. Zool Sci 12:105–112
Yazaki I (2002) Mechanisms of sea urchin metamorphosis: stimuli and responses. In: Yokota Y, Matranga V, Smolenicka Z (eds) The sea urchin: from basic biology to aquaculture. Swets & Zeitlinger, Lisse, pp 51–71
Yazaki I, Harashima H (1994) Induction of metamorphosis in the sea urchin, Pseudocentrotus depressus, using L-glutamine. Zool Sci 11:253–260
Zakeri Z, Bursch W, Tenniswood M, Lockshin RA (1995) Cell death: programmed, apoptosis, necrosis, or other? Cell Death Differ 2:87–96
Acknowledgements
We are grateful to Prof. Yoshinori Ohsumi and Dr. Eiko Oita for their kind suggestions regarding the identification of the morphology of autophagic vacuoles, to Profs. Kiyoaki Kuwasawa and Shinichi Hisanaga for their valuable discussions, and to Dr. Isao Uemura for his technical advice on electron microscopy. We are indebted to members of the Marine and Coastal Research Center of Ochanomizu University and to Profs. Takashi Suyemitsu and Masato Kiyomoto, Ms. Mamiko Yajima and Ms. Ayumi Kikuchi for supplying sea urchin larvae. Our thanks are also due to Dr. Masashi Noguchi for his useful suggestions and for supplying larvae.
Author information
Authors and Affiliations
Corresponding author
Additional information
This investigation was supported in part by a Keio University special grant-in-aid for innovative collaborative research projects.
Rights and permissions
About this article
Cite this article
Sato, Y., Kaneko, H., Negishi, S. et al. Larval arm resorption proceeds concomitantly with programmed cell death during metamorphosis of the sea urchin Hemicentrotus pulcherrimus . Cell Tissue Res 326, 851–860 (2006). https://doi.org/10.1007/s00441-006-0212-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-006-0212-6