Abstract
The features of the coloration of the dorsal and ventral surfaces of the rare deep-sea Richardson’s ray Bathyraja richardsoni (Arhynchobatidae) in the length range from 14 to 146 cm have been studied and its ontogenetic changes have been traced. New data on the coloration of embryos, immature, maturing, and sexually mature individuals are presented. It is shown that variations in the coloration of the dorsal surface are insignificant, while the coloration of the ventral surface can vary significantly. The data obtained can be used in taxonomic and population studies, in the development of keys for species identification, in the preparation of faunal overviews and field guides, to facilitate understanding the features of coloration and its changes in deep-sea animals living in permanent darkness, and to expand knowledge about certain aspects of macro- and microevolution of deep-sea skates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The fauna of deep-sea skates, even in the most studied northern part of the Atlantic Ocean, has not been studied well enough. Many North Atlantic species have been described relatively recently (in the second half of the last century) and are still known from a small number of records (Orlov et al., 2006; Porteiro et al., 2017). These species include the Richardson’s ray Bathyraja richardsoni, which is first described from the waters of New Zealand (Garrick, 1961; Garrick and Paul, 1974). The distribution of this species is in the north and central Atlantic (from northeastern Canada and southwestern Greenland to the Azores and Mauritania) and in the southwestern Pacific in the waters of Tasmania and New Zealand (Ebert and Stehmann, 2013; Kulka et al., 2015; Porteiro et al., 2017), where it is known from scattered records. Richardson’s ray is one of the largest species of deep-sea skates, reaching a length of 175 cm (Sulak et al., 2009; Ebert and Stehmann, 2013). Its abundance is greatest in the area of the Mid-Atlantic Ridge (MAR) (Fossen et al., 2008); this species is one of the North Atlantic skates with the deepest habitation, whose catches are known from depths of 501–3055 m with a maximum occurrence between 2100 and 2400 m (Orlov et al., 2006; Porteiro et al., 2017).
Skates are one of the groups of marine fish that is the most vulnerable to anthropogenic impact (Dulvy and Reinolds, 2002); individual countries and environmental organizations pay increased attention to the protection and conservation of both cartilaginous fish in general and skates in particular (Orlov, 2005, 2008; Cavanagh and Gibson, 2007; Orlov and Shevernitskii, 2008; Orlov and Litvinov, 2010; Goodwin, 2012). According to the International Union for Conservation of Nature (IUCN), Richardson’s ray is classified as “Least Concern” (Kulka et al., 2015), i.e., there are currently no anthropogenic threats to this species.
Although this species is quite numerous in comparison with other deep-sea skates of the North Atlantic, its biology remains insufficiently studied so far (Tempelman, 1973b; Orlov et al., 2006); most published works describe mainly the morphological features of this species (Garrick, 1961; Forster, 1965; Tempelman, 1973a, 1973b; Garrick and Paul, 1974; Stehmann and Merrett, 2001; Orlov et al., 2006, 2010; Orlov, 2011; Orlov and Cotton, 2011). Meanwhile, the published information about Richardson’s ray coloration is scattered and limited. Stemmann and Merrett (2001) described the coloration of five embryos extracted from egg capsules. Previously, data on the coloration of several large mature Richardson’s rays were described for specimens from the waters of New Zealand (Garrick, 1961), the northeastern Atlantic (NEA) (Forster, 1965), and the northwestern Atlantic (NWA) (Tempelman, 1973b). This information was later generalized in regional faunal reports (Sulak et al., 2009; Ebert and Stehmann, 2013). In general, the following general characters were indicated in these studies for the coloration of adult individuals: the dorsal side of the body is uniformly colored from ash to brown, with small light dots or spots and a dark margin on the wings and lower lobes of the pelvic fins (V); the ventral side of the body is noticeably darker than the dorsal side, with a dark margin on the wings and lower lobes of V and the presence of white areas around the mouth, nostrils, and the cloaca, on the tail, and at its base, between the pectoral (P) and V, on the lower lobes of V and the tops of the claspers.
The lack of published data on the coloration of medium-sized Richardson’s rays did not allow us to investigate the patterns of changes in their coloration throughout its life cycle and to identify its intraspecific and ontogenetic variability. Such information is important for taxonomic and population studies as well as for the development of keys for species identification and the preparation of faunal overviews and field guids. In addition, the development of this problem may be of broader fundamental interest in terms of understanding the features of coloration and its changes in deep-sea animals living in permanent darkness as well as contribute to a better understanding of certain aspects of macro- and microevolution (origin, moving, formation of population structures, etc.) of deep-sea skates.
This work aims to analyze all available (of our own and literary) data on the coloration of the Richardson’s ray, allowing us to trace its ontogenetic changes within the entire species range.
MATERIALS AND METHODS
We used 20 specimens of both sexes with a total length from 14 to 146 cm, studied by the first author during 2005–2010 in various museums as a material for the analysis of variations in the coloration of the Richardson’s ray (see Table 1):
Zoological Museum of the University of Bergen, Norway: ZMUB 19528 (TL 142 cm, female, 2534 m), ZMUB 17600 (TL 29 cm, female, 2534 m), ZMUB 19514-3 (TL 78 cm, male, 1911 m), ZMUB 19514-4 (TL 63 cm, female, 1911 m), ZMUB 19522-3 (TL 45 cm, female, 2364 m), ZMUB 19522-1 (TL 55 cm, male, 2364 m), ZMUB 19522-2 (TL 53 cm, female, 2364 m), ZMUB 19535 (TL 53 cm, male, 2534 m), ZMUB 19514-1 (TL 69 cm, female, 2280 m), ZMUB 19514-2 (TL 84 cm, male, 1911 m), ZMUB 19476 (TL 106 cm, female, 2288 m), ZMUB 19448 (TL 101 cm, female, 2534 m), ZMUB 19364 (TL 85 cm, female, 2951 m); all of them were from the waters of the MAR.
National Museum of Natural History, Paris, France: MNHN 1999-1156 (TL 35 cm, male, 1975 m, NEA, east of the Hebrides).
British Museum of Natural History, London, United Kingdom: BMNH 1999.10.1.1 (TL 52 cm, male, 2441 m, depth and place of capture unknown), BMNH 1999.10.1.2 (TL 69 cm, male, 2441 m, depth and place of capture are unknown), BMNH 1999.2.2.1 (TL 22 cm, male, 1541 m, NEA, east of Ireland), BMNH 1999.2.2.2 (TL 25 cm, male, 1541 m, NEA, east of Ireland), BMNH 1999.2.2.3 (TL 24 cm, female, 1541 m, NEA, east of Ireland), BMNH 1999.2.2.4 (18 cm female, 1541 m, NEA, east of Ireland).
In addition, literary data on coloration, photographs or drawings of specimens from the waters of New Zealand (Garrick, 1961), NEA (Forster, 1965) and NWA (Tempelman, 1973a), as well as the Azores Islands (photo by Gui Menezes), were analyzed for comparison.
The analysis of coloration variations was based on the study of freshly caught individuals (MAR), their descriptions from published literature, drawings (New Zealand waters), or photographs (waters of the Canary and Azores Islands, NEA and NWA), and preserved specimens from museum collections (NEA).
The specific features of the sexual maturation of Richardson’s ray have not been studied, and nothing is known about the size at which it matures. Based on published data, as well as data on the ratio of the length of the clasper to the lower lobe V (Orlov, 2006) in the studied males, it can be concluded that the length of embryos is 14–25 cm, immature animals is 35–69 cm, maturind skates is 78–85 cm, and sexually mature is over 113 cm. At the same time, the length at which males and females of large North Pacific and South African skates mature hardly differs; they reach sexual maturity at 60% of the maximum length (Orlov, 2006; Ebert et al., 2007; Orlov and Smirnov, 2015). In this regard, to indicate the degree of sexual maturity of individuals of the Richardson’s ray in the studied size range, when discussing the results, we conditionally distinguish the following groups: embryos (18–25 cm), immature (29–69 cm), maturing and sexually mature (78–103 cm), sexually mature (above 105 cm, it is 60% of the maximum known length).
RESULTS
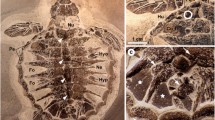
The results of the analysis are presented in Table 1 that includes 13 characters (features of coloring). The analysis does not include an early embryo with a length of 142 mm BMNH 1999.2.2.4 (Fig. 1), which was colored in one color and did not have the characteristic features of the coloration both in fresh (Stehmann and Merrett, 2001) and in a preserved condition (our data). The description of the coloration is illustrated by photographs of skates representing the size range 14, 18, 25, 35, 52, 69, 84, 106, and 127 cm (Figs. 1–9).
Dorsal Surface of the Body
Variations in the color of the dorsal surface of the Richardson’s ray in comparison with those of the ventral can be considered insignificant. For all the studied individuals of this species (excluding specimens that lacked data on certain characters), regardless of their size, sex, and state of maturity, the monotonous coloring of the dorsal side from ash to brown was characteristic (see Table 1). The vast majority of individuals had a darker margin along its edge and on the lower lobes of V (96.4%) as well as small light dots or spots on the dorsal surface of the disc (96.4%) (Fig. 2). In this respect, a 110-cm-long female (no. 22 in Table 1) from the waters of the Canary Islands (Brito et al., 1998), in which (judging by the photo) both of the considered characters were absent, was an exception.
Ventral Surface of the Body
Variations in the coloration of the ventral surface of Richardson’s ray were more diverse. In all the analyzed skates, without exception, the ventral surface, in general, was ash-brown, regardless of length, sex, and sexual maturity. At the same time, it was usually darker than the dorsal surface, and there was a dark margin on the edges of the wings and lower V lodes. Also, all the studied individuals were characterized by the presence of white areas around the mouth, nostrils, and from the nostrils to the corners of the mouth (Fig. 3). In the vast majority of individuals (92.3%), the cloaca opening was white (see Fig. 3), except for two 18- and 22‑cm-long embryos (nos. 1, 2 in Table 1 and Figs. 4b, 6b). The underside of the tail was completely white in two embryos, a TL 18 cm female and a TL 35 cm male (nos. 1, 6, respectively). In other individuals, the ventral surface of the tail is pigmented at varying degrees (to completely dark, Figs. 3, 5); moreover, some of them had a white area at its base (Figs. 4b, 6). Approximately one third of the studied specimens (35.7%) were characterized by the presence of light spots on the tail (Fig. 8b), except for the two individuals already mentioned above, whose tail was completely white on the ventral side.
In 73.1% of individuals, a white patch at the junction of the P and V (Figs. 3, 4b, 6b, 7b, 8b, 9b) was noticed, except for seven females and males, ranging from 29 to 146 cm (Fig. 5b). In a slightly larger number of individuals (76.9%), white areas on the lower lobes of V are noticed (Figs. 4b, 9b), except for six males and females with the length from 69 to 113 cm (Figs. 5b, 8b). Slightly more than one third of the studied specimens with a length up to 69 cm had white spots on the belly (Fig. 8b), the shape, location, and size of which varied. Embryos with a length of 18, 22, and 24 cm retained light-colored areas in the place of the yolk sac (Figs. 4b, 9b), and there were also additional light areas around the gill slits (Fig. 9b). The 24‑cm-long embryo (no. 3) had additional light areas forming an arrow-like shape, with the apex at the attachment point of the yolk sac and the base at the lower V lobes (Fig. 9b). In a 35-cm-long male (no. 6), a white spot is noticed between the fifth gill slits (Fig. 6b). The 69-cm-long male (no. 14) had a white spot at the level between the second and fifth gill slits (Fig. 7b). In addition, 80.0% of males had white patches on the claspers (Figs. 3, 4b, 5b, 6b, 7b), except for 53-, 69-, and 78-cm-long specimens (nos. 10, 14, 16).
Features of Coloration of Various Size Groups
In general, regardless of size and sex (except for the TL 14 cm embryo), all the studied individuals had a monotone dorsal color, from ash to brown with minor variations in the form of the presence of a darker margin on the edges of P and V as well as small dots and spots on the disc. Variations in the color of the ventral surface of the body were more significant, although all the studied specimens were characterized by its darker color in comparison with the dorsal surface and the presence of a dark margin on the wings and lower lobes of V.
Embryos. Our detailed studies of embryos previously studied by Stehmann and Merrett (2001) in a preserved condition (nos. 1–4 Table 1) revealed some additional features of their coloration. The 14-cm-long embryo had a monotonic pale color both in a preserved and freshly caught state (Stehmann and Merrett, 2001). Two other embryos, TL 18 cm (no. 1) and TL 22 cm (no. 2), lacked a white spot at the entrance of the cloaca. In the first of these specimens, in addition, the entire ventral surface of the tail was white, unlike most specimens, in which a light area is noticed only for the base of the tail. Four of the specimens under consideration had white spots on the belly of various configurations and locations, which were absent in skates with a size over 85-cm-long. Among the embryos, in all males, the dorsal surface of the claspers was light.
Immature specimens. According to our data, all immature individuals have white areas around the mouth, nostrils, at the entrance to the cloaca, and the base of the tail. At the same time, some features of their coloration were markedly different from those of maturing and sexually mature specimens. Thus, some of the individuals, 29–69 cm of TL (four out of 11 specimens) lacked a white area between P and V in the presence of it in all individuals of shorter length and most larger specimens. Two 69-cm-long rays (nos. 14, 15) have no white areas on the lower V lobes. Four individuals, TL 52–55 cm (nos. 8–11) and TL 69 cm (no. 14) had no white spots on the belly, which is present in most of the maturing and sexually mature specimens. Skates TL 55 cm (no. 11) and TL 69 cm (no. 14) have no white spots on the tail. Two males, TL 53 cm (no. 10) and TL 69 cm (no. 14) lacked white patches on the tops of the claspers, which are present in all smaller and most of larger specimens.
Maturing specimens. The coloration of the ventral surface of the body of all maturing individuals was characterized by the presence of white areas around the mouth, nostrils, and entrance to the cloaca. One of the five studied specimens (no. 16) lacked a white spot between P and V. Three of the five individuals (nos. 16–17, 19) had no white areas on the lower lobes of V. No maturing specimens had white spots on the belly, except for the TL 84 cm male (no. 17). No skates of the group under consideration had white spots on the tail. Of the two maturing males that were studied (nos. 16, 17), the tops of the claspers were light-colored in only one.
Mature specimens. Like the vast majority of individuals of other size groups, mature specimens have white areas around the mouth and nostrils and at the entrance to the cloaca. Three of the seven skates studied (nos. 21, 23–24) had white patches at the base of the tail. The presence of white areas between P and V was noted in two (nos. 23, 28) of the seven specimens. The lower lobes of V were light-colored in only one individual (no. 23). White spots on the belly and ventral surface of the tail are not noticed for any sexually mature skate. At the same time, in all studied males of this group, the tops of the claspers were light-colored.
Variations of Coloration Depending on Sex
The analysis of variations in the coloration of the ventral surface of the body of 14 females and 12 males did not reveal its fundamental differences in individuals of different sexes. The absence of a white coloring at the area of the entrance to the cloaca was noted in two of the smallest embryos of different sex (nos. 1, 2). White areas at the base of the tail were absent in one large female (no. 21) and four males, TL 78–127 cm (nos. 16, 17, 23–24). There were no white areas between P and V in three females, TL 29, 45, and 146 cm (nos. 5, 7, 28) and four males, TL 55, 69, 78, 113 cm (nos. 11, 14, 16, 23). White areas on the lower lobes of the V are not marked in two females, TL 69 and 101 cm (nos. 15, 19), and four males, TL 69–113 cm (nos. 14, 16–17, 23). White spots on the belly were absent in eight females, TL 53 cm and TL > 85 cm, while this character among males was not expressed in specimens TL 52–78 cm and TL 113–130 cm. White spots on the tail were absent in seven females TL > 85 cm and seven males TL > 55 cm.
Variations of Coloration Depending on the Depth
In individuals of Richardson’s ray caught at various depths in the range of 1541–2951 m, some differences in the coloring features of the ventral surface of the body were noted. In the course of this analysis, the specified range was divided into three strata: 1541–1975 m (eight individuals), 2125–2441 m (13 individuals), and 2534–2951 m (five individuals). The skates with a lack of white areas at the entrance to the cloaca were found only at depths <2000 m. White areas at the base of the tail were absent in two specimens from depths <2000 m and in three specimens from 2000–2500 m but were not noted in individuals from >2500 m. White areas between P and V were absent in two skates caught at a depth of <2000 m, in four rays from 2000–2500 m, and one from >2500 m. On the lower lobes of V, white areas were not found in three individuals from depths <2000 m, in two from 2000–2500 m, and in one from >2500 m. White spots on the belly were absent in two specimens that were caught at depths <2000 m, in ten that were caught at 2000–2500 m, and in four that were caught at >2500 m. White spots on the tail were absent in three specimens from depths <2000 m, in eight specimens from 2000–2500 m, and in three specimens from >2500 m. The absence of white tops of claspers was found in only two males from depths less than 2000 m and in one from over 2500 m.
Variations of Coloration Depending on the Area
The small number of specimens from the waters of New Zealand (one specimen) and NWA (two specimens) did not allow us to use them in comparative analysis. Larger samples (nine specimens) from the waters of the NEA, MAR, and Azores Islands (14 specimens) provide an opportunity to compare the color of the ventral surface of the body of considered species in these areas. Individuals with the absence of white area at the entrance to the cloaca (two specimens) were found only in the NEA. The absence of the white area at the base of the tail was more typical for skates from the MAR waters (four specimens) than from the NEA (one specimen). Also, individuals from the waters of the MAR more often lacked white areas between P and V (four specimens) in comparison with NEA (two specimens) as well as on the lower lobes of V (five and one specimens, respectively). White spots on the belly were absent in ten rays from the MAR waters and in three rays from the NEA. The absence of white spots on the tail was noted in nine individuals from the waters of the MAR and in two from the NEA. Also, we should note that all the analyzed skates from the waters of New Zealand and the NWA also did not have white spots on the belly and tail. The presence of white tops of claspers was noted only in three males from the waters of MAR.
DISCUSSION
The information about the coloration of Richardson’s ray available in the literature is limited to the description of embryos (Stehmann and Merrett, 2001) and adult mature individuals (Garrick, 1961; Forster, 1965; Tempelman, 1973a; Sulak et al., 2009; Ebert and Stehmann, 2013). Data on the coloration features of immature and maturing individuals of this species have not been available in published sources so far, except for a female from the MAR region (Orlov, 2011), which has some anomalies in the external morphology and coloration. Thus, the information available in the literature did not make it possible to describe the ontogenetic changes in the coloration of the Richardson’s ray in full, although there were references to such description in the previously published literature (Orlov and Cotton, 2011).
Describing the coloration of embryos extracted from egg capsules, with TL 14–25 cm, Stehmann and Merrett (2001) noted that it is generally similar to that of adult individuals, but the coloring of the dorsal and ventral surfaces in adult animals is more different than in embryos. At the same time, the lower surface is noticeably darker in embryos, while the color differences of the dorsal and ventral surfaces in adults are not so pronounced. Our studies of the coloration of preserved embryos did not find any noticeable differences between the dorsal and ventral surfaces; however, we found additional features of the latter that were not mentioned earlier and that are absent in adults.
Until now, there was no information about the coloring features of medium-sized (immature and maturing) Richardson’s rays. Our data on the coloration of medium-sized Richardson’s rays significantly supplement the available information and allow us to trace the ontogenetic changes in the coloration of this species in full.
There is enough information about the coloration of adult sexually mature individuals of the Richardson’s ray in the published literature (Garrick, 1961; Forster, 1965; Tempelman, 1973a; Sulak et al., 2009; Ebert and Stehmann, 2013). The main color of the dorsal surface, according to our data, did not differ in principle from the one described earlier (Garrick, 1961; Forster, 1965; Tempelman, 1973a; Sulak et al., 2009; Ebert and Stehmann, 2013). We also noted the presence of small light dots and spots on the dorsal surface as well as a dark margin on the edges of the disc and the lower lobes V in most of the analyzed individuals, except for a 110-cm-long female (no. 22) from the waters of the Canary Islands (Brito et al., 1998). The presence of white areas around the mouth and nostrils has been reported by many authors (Garrick, 1961; Forster, 1965; Tempelman, 1973b). Our data on large skates fully correspond to these observations.
The results of our analysis allow us to significantly clarify certain features of the coloration of the ventral surface of large specimens of the Richardson’s ray.
The presence of a white area at the base of the tail has been noted by several authors (Garrick, 1961; Forster, 1965; Tempelman, 1973b; Sulak et al., 2009; Ebert and Stehmann, 2013). However, some authors indicate the presence of a white area at the base of the tail, others indicate the presence of white stripes (spots) on the tail. According to our data, both of these characters should be considered independent. The presence of a white spot at the base of the tail is not typical for all large skates since it is not found in individuals with a length of 106–127 cm (nos. 21, 23–24), and white spots on the tail are found mainly in young specimens.
The presence of a spot at the entrance of cloaca was previously noted by several authors (Garick, 1961; Foerster, 1965; Tempelmann, 1973b). According to our data, this feature is characteristic of the coloration of all large skates of this species.
Another feature that is typical for the coloration of adult Richardson’s rays is the presence of a white area between P and V (Garrick, 1961; Tempelman, 1973b). According to our data, this character was absent in a 113-cm-long male (no. 23) and a 146-cm-long female (no. 28).
Some authors (Sulak et al., 2009; Ebert and Stehmann, 2013) pay attention to the presence of white areas on the lower V lobes. Among the large individuals we studied, this character was absent in only a 101-cm-long female (no. 19) and a 113-cm-long male (no. 23).
Few authors (Garrick, 1961; Tempelman, 1973b) have pointed out the presence of white areas around the gill slits, which are practically invisible in the above images. The only drawing of the holotype (Garrick, 1961) clearly shows narrow white areas around the gill slits. Our studies have not revealed the presence of white areas around the gill slits in the studied large specimens.
Another feature that is characteristic of large males of the Richardson’s ray is the white tops of the claspers (Tempelman, 1973b). Our studies have shown that this character is well expressed in large (over 84-cm-long) males.
The last feature of the coloration of large individuals, which is mentioned in the published literature, is the light areas located along the median line of the body (Sulak et al., 2009; Ebert and Stehmann, 2013). What these sites are, these sources do not explain. We assume that we may be talking in this case about white spots on the belly in the central part of the disc, which, according to our data, may have different locations and configurations (see above) in individuals of different lengths as well as white areas at the base of the tail and along it.
Thus, the analysis of literature and our own data shows that individual features of Richardson’s ray coloration persist throughout the entire life cycle and hardly depend on size, gender, depth, and area of habitation. These include the color of both surfaces of the body, the presence of light dots or spots on the dorsal surface of the disc, the presence of a dark margin on the wings, and lower V lobes on both sides of the body and white areas around the mouth and nostrils.
Almost all individuals (TL > 24 cm) have a white area at the entrance to the cloaca; it is absent only in the smallest embryos (nos. 1–2). Most of the individuals have a white patch at the base of the tail, while it is absent in several maturing (nos. 16–17) and sexually mature (nos. 21, 23–24) animals. Among immature skates, unlike embryos, specimens appear (nos. 5, 7, 11, 14), in which there are no white areas between P and V, which is also occasionally observed in maturing (no. 16) and sexually mature (nos. 23, 28) skates. White patches on the lower lobes of the V disappear in immature individuals, with TL > 69 cm (nos. 14–15), which was also noted in most of the maturing ones (nos. 16–17, 19) and one sexually mature specimen (no. 23). White spots on the belly disappear in immature individuals, with TL > 52 cm (exception is no. 13, TL 63 cm) and are not found among both maturing (no. 17, TL 84 cm) and sexually mature skates. White spots on the tail are characteristic only for young specimens, they disappear at TL > 55 cm, and are not observed in mature and sexually mature individuals (except for no. 14, TL 69 cm). The presence of white patches on the claspers is characteristic of most of the studied males, except for two immature (nos. 10, 14) and one maturing (nos. 16) specimens. Throughout the life cycle, this feature is subject to significant changes: the ventral surface of the claspers is entirely light in embryos and immature males, but only the tops of the claspers remain light-colored in maturing and sexually mature animals.
In general, the males and females of the Richardson’s ray did not differ fundamentally from each other in coloration. However, four characters by which the coloration of individuals of different sexes had noticeable differences can be distinguished: a white area at the base of the tail (92.9 vs. 66.7% in females and males, respectively), a white area between P and V (78.6 vs. 66.7%), a white area on the lower lobes of V (85.8 vs. 66.7%), and a white spot on the belly (42.9 vs. 33.3%). It should be noted that the four features considered in individuals of different sexes were manifested in different ways. The white area at the base of the tail was found in all females (exception is no. 21, TL 106 cm), but it was not noted in all large males with TL > 78 cm (exception is no. 25, TL 130 cm). Almost all large females with TL > 103 cm had white patches on the lower lobes of V (without exception) and between P and V (except no. 28, TL 146 cm), while these features were not expressed in more than half of large males with TL > 55 cm (57.1%). The presence of white spots on the belly was noted mainly for young specimens of both sexes. It can be assumed that the detected differences in the coloration of large males and females help to facilitate the search for a sexual partner in the situation of low abundance of the species in conditions of complete absence of light.
It was not possible to identify fundamental differences in the color features of the Richardson’s ray depending on the depth. It can only be noted that the embryos that lacked white areas at the entrance to the cloaca were found only at depths <2000 m. This might be explained by the fact that there are no their records at other depths yet. Among the skates that were caught at a depth of >2500 m, no animals were lacking a white area at the base of the tail. White patches on the tops of claspers were present in all skates caught at depths of 2000–2500 m, unlike specimens from greater depths and shallower habitations. The presence or absence of other studied features was noted in individuals regardless of the depth of capture. Most likely, the detected coloration differences at different depths are random since this species does not show ontogenetic vertical migrations, unlike other deep-sea skates, for example, from the North Pacific (Orlov et al., 2006b), and the size of individuals is not related to the depth of capture (Orlov et al., 2006a).
Fundamental differences in the coloration of the Richardson’s ray from different areas were not found. Nevertheless, in some characters, individuals from the waters of the MAR and NEA were different. Thus, the frequency of occurrence was, respectively, 71.4 and 88.9% for white areas at the base of the tail, 64.3 and 77.8% for white areas between P and V, 64.3 and 88.9% for white areas on the lower lobes of V, 28.6 and 66.7% for white spots on the belly, 28.6 and 77.8% for white spots on the tail, and 50 and 100% for white tops of claspers. It is not yet possible to explain the reasons for the detected geographical differences in the coloration of individuals of the Richardson’s ray. However, there is information about the specific coloration in the waters of the MAR and NEA of other deep-sea skates as well, for example, Jensen’s skate Amblyraja jenseni, pale ray Bathyraja pallida, and Mid-Atlantic skate Rajella kukujevi (Orlov and Cotton, 2013, 2015; Orlov, 2014).
The coloration of freshly caught and formalin-preserved specimens of Richardson’s ray varies, but very slightly. Garrick (1961) pointed out that the coloration of individuals becomes a bit darker after preservation in formalin. Templeman (1973b) believed that half of the individuals do not change their color after preservation and the ventral surface becomes darker in half of them, especially along the edges of the disc. Our results showed that the color intensity of the dorsal and ventral surfaces of the embryos hardly differ after preservation. Our studies of deep-sea North Atlantic skates (Orlov and Cotton, 2013, 2015; Orlov, 2014; present report), based on the study of both freshly caught and preserved samples, show that both are quite suitable for analyzing the features of their coloration.
CONCLUSIONS
The information on the presence of species-specific coloration of the ventral surface in deep-sea North Atlantic skates given in this article and previously published raises a legitimate question about its biological meaning in species living in conditions of the permanent absence of light. In our opinion, this phenomenon is an evolutionary acquisition aimed at facilitating the search for a sexual partner in conditions of low population. It is known that mating in various species of the superorder Batoidea is preceded by the positioning of one partner over the other, followed by male capturing the edge of the female’s wing with by its jaws (Tricas, 1980; Luer and Gilbert, 1985; Yano et al., 1999; Chapman et al., 2003). It is not known how the mating process occurs in deep-sea skates, but it is unlikely that it is fundamentally different from the above-described process. In conditions of complete darkness and low density of population, the success of each mating attempt is of great importance for the survival of the species. Therefore, reproductive success largely depends on the correct species-specific identification of each other by partners. Probably, the species-specific features of the coloration of the ventral surface of the body of deep-sea skates serve as a kind of marker in the “in-group and out-group” identification system, which allows sexual partners to accurately determine not only belonging to the same species but also readiness for mating. This hypothesis raises a legitimate question: how do sexual partners distinguish the pattern on the ventral surface of the body? It is known that various species of Rajiformes can emit weak electric signals (Baron et al., 1992; Baron, 1994; New, 1994; Sincere et al., 1998; Sincere and Tricas, 2002), using them, among other things, to find a sexual partner (Tricas et al., 1995). It can be assumed that deep-sea skates, in search of a suitable object for mating, use a similar mechanism that allows them to distinguish the features of the pattern on the ventral surface of the body. However, the role of vision in this process should not be excluded, since the presence of visual pigment in the eyes of deep-sea species, including Richardson’s ray (Douglas et al., 1995; Lisney et al., 2012), indicates that it plays an important role in the life of this species.
REFERENCES
Baron, V.D., Possible role of electroreception in the behavior of low-electric rays Raja clavata (Rajidae), Sensor. Sist., 1994, vol. 8, nos. 3–4, pp. 147–161.
Baron, V.D., Sokolova, L.S., and Mikhailenko, N.A., Identification of spinal electromotoneurons in the ray Raja clavata (Rajidae), Neuroscience, 1992, vol. 48, no. 2, pp. 397–403.
Brito, A., Pascual-Alayón, P.J., Rabanal, R., et al., Peces Cartilaginosos de Canarias. Los Tiburones de los Fondos Profundos y su Aprovechamiento Pesquero, Santa Cruz de Tenerife: Consejeria de Agricultura, Pesca y Alimentacion. Gobierno de Canarias, 1998.
Cavanagh, R.D. and Gibson, C., Overview of the Conservation Status of Cartilaginous Fishes (Chondrichthyans) in the Mediterranean Sea, Gland, Switzerland and Malaga, Spain: IUCN Species Survival Commission Shark Specialist Group, 2007.
Chapman, D.D., Corcoran, M.J., Harvey, G.M., et al., Mating behavior of southern stingrays, Dasyatis americana (Dasyatidae), Environ. Biol. Fish., 2003, vol. 68, no. 3, pp. 241–245.
Douglas, R.H., Partridg, J.C., and Hope, A.J., Visual and lenticular pigments in the eyes of demersal deep-sea fishes, J. Comp. Physiol. A, 1995, vol. 177, no. 1, pp. 111–122.
Dulvy, N.K. and Reynolds, J.D., Predicting extinction vulnerability in skates, Conserv. Biol., 2002, vol. 16, no. 2, pp. 440–450. https://doi.org/10.1046/j.1523-1739.2002.00416.x
Ebert, D.A. and Stehmann, M.F.W., Sharks, batoids and chimaeras of the North Atlantic, FAO Spec. Catalogue Fish. Purpose, Rome: FAO, 2013, no. 7.
Ebert, D.A., Compagno, L.J., and Cowley, P.D., Aspects of the reproductive biology of skates (Chondrichthyes: Rajiformes: Rajoidei) from southern Africa, ICES J. Mar. Sci., 2008, vol. 65, no. 1, pp. 81–102. https://doi.org/10.1093/icesjms/fsm169
Forster, G.R., Raja richardsoni from the continental slope off south-west England, J. Mar. Biol. Assoc. U.K., 1965, vol. 45, pp. 773–777. https://doi.org/10.1017/S0025315400016581
Fossen, I., Cotton, C.F., Bergstad, O.A., and Dyb, J.E., Species composition and distribution patterns of fishes captured by longlines on the Mid-Atlantic Ridge, Deep-Sea Res. II, 2008, vol. 55, pp. 203–217. https://doi.org/10.1016/j.dsr2.2007.09.004
Garrick, J.A.F., Studies on New Zealand Elasmobranchii. Part XIII. A new species of Raja from 1,300 fathoms, Trans. R. Soc. N.Z., 1961, vol. 88, pt. 4, pp. 743–748.
Garrick, J.A.F. and Paul, L.J., The taxonomy of New Zealand skates (suborder Rajoidea), with descriptions of three new species, J. R. Soc. N.Z., 1974, vol. 4, no. 3, pp. 345–377. https://doi.org/10.1080/03036758.1974.10419402
Goodwin, H.B., Skate and ray management in the Northwest Atlantic: an overview of current management and recommendations for conservation, MS Thesis, Halifax: Dalhousie Univ., 2012.
Kulka, D.W., Orlov, A., and Barker, A.S., Bathyraja richardsoni, The IUCN Red List of Threatened Species, 2015. e.T63127A70709214. Accessed May 19, 2020.https://doi.org/10.2305/IUCN.UK.2015-4.RLTS.T63127A70709214.en
Lisney, T.J., Theiss, S.M., Collin, S.P., and Hart, N.S., Vision in elasmobranchs and their relatives: 21st century advances, J. Fish. Biol., 2012, vol. 80, no. 5, pp. 2024–2054. https://doi.org/10.1111/j.1095-8649.2012.03253.x
Luer, C.A. and Gilbert, P.W., Mating behavior, egg deposition, incubation period, and hatching in the clearnose skate, Raja eglanteria, Environ. Biol. Fish., 1985, vol. 13, no. 3, pp. 161–171.
New, J.G., Electric organ discharge and electrosensory reafference in skates, Biol. Bull., 1994, vol. 187, no. 1, pp. 64–75.
Orlov, A.M., Results of the meeting of FAO and WWF specialists “Conservation and Management of Deep-Sea Cartilaginous Fish Fishery”, Biol. Morya, 2005, vol. 31, no. 1, pp. 68–70.
Orlov, A.M., On the substantiation of the commercial harvest measure of the Far Eastern rays (family Rajidae) using massive Western Bering Sea species as an example, Tr. VNIRO, 2006, vol. 146, pp. 252–264.
Orlov, A.M., Red List of the World Conservation Union and the Conservation Status of the Chondrichthyes of the Western Part of the Northern Pacific, J. Ichthyol., 2008, vol. 48, no. 6, pp. 476–478.
Orlov, A.M., Record of tailless Richardson’s ray Bathyraja richardsoni (Garrick, 1961) (Rajiformes: Arhynchobatidae) caught off the Mid-Atlantic ridge, Pan-Am. J. Aquat. Sci., 2011, vol. 6, no. 3, pp. 232–236.
Orlov, A.M., New data on rare deep-water Mid-Atlantic skate Rajella kukujevi (Rajidae), J. Ichthyol., 2014, vol. 54, no. 5, pp. 317–337. https://doi.org/10.1134/S0032945214030102
Orlov, A.M. and Cotton, C.F., Sexually dimorphic morphological characters in five North Atlantic deepwater skates (Chondrichthyes: Rajiformes), J. Mar. Biol., 2011, vol. 2011, art. ID 842821. https://doi.org/10.1155/2011/842821
Orlov, A.M. and Cotton, C.F., New data on rare deepwater North Atlantic skate Bathyraja pallida (Forster, 1967) (Arhynchobatidae, Rajiformes), J. Ichthyol., 2013, vol. 53, no. 7, pp. 465–477. https://doi.org/10.1134/S0032945213040048
Orlov, A.M. and Cotton, C.F., New data on the rare deep-sea skate Amblyraja jenseni (Rajidae) from the North Atlantic ocean, J. Ichthyol., 2015, vol. 55, no. 4, pp. 478–496. https://doi.org/10.1134/S0032945215040086
Orlov, A.M. and Litvinov, F.F., International efforts to assess the conservation status of cartilaginous fish in the World Ocean, Tr. VNIRO, 2010, vol. 149, pp. 92–114.
Orlov, A.M. and Shevernitskii, D.A., Nature-conservation status of cartilaginous fishes of the North-East Atlantic, Byull. Mosk. O-va Ispyt. Prir., Otd. Biol., 2008, vol. 113, no. 4, pp. 27–32.
Orlov, A.M. and Smirnov, A.A., New data on sexual dimorphism and reproductive biology of Alaska skate Bathyraja parmifera from the Northwestern Pacific Ocean, J. Ichthyol., 2011, vol. 51, no. 8, pp. 590–603.
Orlov, A., Cotton, C., and Byrkjedal, I., Deepwater skates (Rajidae) collected during the 2004 cruises of R.V. “G.O. Sars” and M.S. “Loran” in the Mid-Atlantic Ridge area, Cybium, 2006a, vol. 30, no. 4 (suppl.), pp. 35–48.
Orlov, A., Tokranov, A., and Fatykhov, R., Common deep-benthic skates (rajidae) of the northwestern pacific: basic ecological and biological features, Cybium, 2006b, vol. 30, no. 4 (suppl.), pp. 49–65.
Orlov, A.M., Cotton, C.F., and Shevernitsky, D.A., Sexual dimorphism of external morphological characters in some deepwater skates (Rajidae, Rajiformes, Chondrichthyes) of the North Atlantic, Moscow Univ. Biol. Sci. Bull., 2010, vol. 65, no. 1, pp. 40–44. https://doi.org/10.3103/S0096392510010086
Porteiro, F.M., Sutton, T.T., Byrkjedal, I., et al., Fishes of the Northern Mid-Atlantic Ridge collected during the MAR-ECO cruise in June–July 2004: an annotated checklist, Arquipelago. Life Mar. Sci. Suppl., 2017, Suppl. 10.
Sisneros, J.A. and Tricas, T.C., Ontogenetic changes in the response properties of the peripheral electrosensory system in the Atlantic stingray (Dasyatis sabina), Brain Behav. Evol., 2002, vol. 59, no. 3, pp. 130–140.
Sisneros, J.A., Tricas, T.C., and Luer, C.A., Response properties and biological function of the skate electrosensory system during ontogeny, J. Comp. Physiol. A, 1998, vol. 183, no. 1, pp. 87–99.
Stehmann, M.F.W. and Merrett, N.R., First records of advanced embryos and egg capsules of Bathyraja skates from the deep north-eastern Atlantic, J. Fish. Biol., 2001, vol. 59, pp. 338–349. https://doi.org/10.1111/j.1095-8649.2001.tb00134.x
Sulak, K.J., MacWhirter, P.D., Luke, K.E., et al., Identification guide to skates (family Rajidae) of the Canadian Atlantic and adjacent regions, Can. Tech. Rept. Fish. Aquat. Sci., 2009, no. 2850.
Tempelman, W., The skate, Raja richardsoni Garrick, 1961, assigned to Bathyraja, J. Fish. Res. Board Can., 1973a, vol. 30, pp. 1729–1732.
Tempelman, W., First records, description, distribution, and notes on the biology of Bathyraja richardsoni (Garrick) from the Northwest Atlantic, J. Fish. Res. Board Can., 1973b, vol. 30, pp. 1831–1840.
Tricas, T.C., Courtship and mating-related behaviors in myliobatid rays, Copeia, 1980, no. 3, pp. 553–556.
Tricas, T.C., Michael, S.W., and Sisneros, J.A., Electrosensory optimization to conspecific phasic signals for mating, Neurosci. Lett., 1995, vol. 202, nos. 1–2, pp. 129–132.
Yano, K., Sato, F., and Takahashi, T., Observations of mating behavior of the manta ray, Manta birostris, at the Ogasawara Islands, Japan, Ichthyol. Res., 1999, vol. 46, no. 3, pp. 289–296.
ACKNOWLEDGMENTS
We thank our colleagues Ingvar Byrkjedal (Zoological Museum, University of Bergen, Bergen, Norway), Patrick Campbell and Roberto Miguez, (British Museum of Natural History, London, Great Britain), and Bernard Séret and Romain Causse (Museum National d’Histoire Naturelle, Paris, France) who made it possible to study materials from collections and provided facilities and equipment for this study. Charles Cotton (Savannah State University, Savannah, United States) provided assistance in measuring ZMUB specimens. Gui Menezes (Departamento de Oceanografia e Pescas, Universidade dos Açores, Horta, Portugal) provided photographs of specimens from the waters of the Azores Islands. We are also grateful to anonymous reviewers for valuable comments and suggestions that have significantly improved the quality of the article.
Funding
The preparation of this work was carried out within the framework of the State Assignment of the Ministry of Education and Science of the Russian Federation no. 0128-2021-0008. The collection of the materials at sea and study of specimens in various museums by the first author were conducted within the international project “MAR-ECO.”
Author information
Authors and Affiliations
Contributions
A.M. Orlov: primary data collection, generation of database, idea formulation, writing of the manuscript; N.I. Rabazanov: development of data analysis methodology, interpretation of results, editing of the manuscript; A.I. Nikiforov: data analysis, preparation and editing of illustrations, editing of the manuscript. All the authors participated in the discussion of the results.
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflict of interests.
Statement on the welfare of animals. All applicable international, national, and/or institutional principles for the use of animals in experiments and conditions of animal care have been followed.
Additional information
Translated by A. Ermakov
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Orlov, A.M., Rabazanov, N.I. & Nikiforov, A.I. Ontogenic Changes in Coloration of Rare Deepwater Richardson’s Ray Bathyraja richardsoni (Arhynchobatidae, Rajiformes, Chondrichthyes). Russ J Dev Biol 53, 27–40 (2022). https://doi.org/10.1134/S1062360422010052
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062360422010052