Abstract
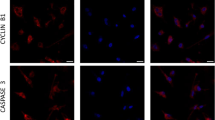
In the mammalian ovary, serotonin has all the components necessary for its signaling function and affects the functional activity of granulosa cells. At the same time, the accumulation of serotonin with the help of a specific SERT transporter occurs mainly in the oocytes of growing ovarian follicles and is hardly present in granulosa cells. Quantitative analysis of mRNA expression of markers of proliferation, apoptosis, and functional state of follicular cells, as well as oocyte growth factors, was performed on an experimental model of ovarian follicle culture. Serotonin (1 µM) does not exhibit pronounced mitogenic and pro- and antiapoptotic properties and does not affect the expression of steroidogenesis markers. At the same time, serotonin stimulates the expression of cyclin genes Ccnd1, Ccnd2, and Ccne1 and also Has2, Ptgs2, Ptgfr, Igfbp, and Ihh in granulosa cells. Also, the addition of serotonin leads to an increase in the expression of Gdf9 in oocytes. There is a more pronounced effect of serotonin compared to the primary culture of granulosa cells, which in all cases, with the exception of cyclins, is canceled by fluoxetine (10 µM). The results obtained indicate that the functional activity of granulosa cells is regulated by serotonin through its effect on the oocyte and is mediated by SERT activity.




Similar content being viewed by others
REFERENCES
Amireault, P., Sibon, D., and Côté, F., Life without peripheral serotonin: insights from tryptophan hydroxylase 1 knockout mice reveal the existence of paracrine/autocrine serotonergic networks, ACS Chem. Neurosci., 2013, vol. 4, no. 1, pp. 64–71.
Azmitia, E.C., Modern views on an ancient chemical: serotonin effects on cell proliferation, maturation, and apoptosis, Brain Res. Bull., 2001, vol. 56, no. 5, pp. 413–424.
Bader, M., Serotonylation: serotonin signaling and epigenetics, Front. Mol. Neurosci., 2019, vol. 12, p. 288.
Bao, B., Garverick, H.A., Smith, G.W., et al., Changes in messenger ribonucleic acid encoding luteinizing hormone receptor, cytochrome P450-side chain cleavage, and aromatase are associated with recruitment and selection of bovine ovarian follicles, Biol. Reprod., 1997, vol. 56, no. 5, pp. 1158–1168.
Bertoli, C., Skotheim, J.M., and Bruin, R.A.M.De., Control of cell cycle transcription during G1 and S phases, Nat. Rev. Mol. Cell Biol., 2015, vol. 14, no. 8, pp. 518–528.
Bòdis, J., Bognàr, Z., Hartmann, G., et al., Measurement of noradrenaline, dopamine and serotonin contents in follicular fluid of human graafian follicles after superovulation treatment, Gynecol. Obstet. Invest., 1992, vol. 33, no. 3, pp. 165–167.
Buznikov, G.A., Preneural transmitters as regulators of embryogenesis. Current state of problem, Russ. J. Dev. Biol., 2007, vol. 38, no. 4, pp. 213–220.
Diaz, F.J., Wigglesworth, K., and Eppig, J.J., Oocytes determine cumulus cell lineage in mouse ovarian follicles, J. Cell Sci., 2007, vol. 120, no. 8, pp. 1330–1340.
Dubé, F. and Amireault, P., Local serotonergic signaling in mammalian follicles, oocytes and early embryos, Life Sci., 2007, vol. 81, nos. 25–26, pp. 1627–1637.
Farrelly, L.A., Thompson, R.E., Zhao, S., et al., Histone serotonylation is a permissive modification that enhances TFIID binding to H3K4me3, Nature, 2019, vol. 567, no. 7749, pp. 535–539.
Gui, L.-M. and Joyce, I.M., RNA interference evidence that growth differentiation factor-9 mediates oocyte regulation of cumulus expansion in mice, Biol. Reprod., 2005, vol. 72, no. 1, pp. 195–199.
Kidder, G.M. and Vanderhyden, B.C., Bidirectional communication between oocytes and follicle cells: ensuring oocyte developmental competence, Can. J. Physiol. Pharmacol., 2010, vol. 88, no. 4, pp. 399–413.
Koppan, M., Bodis, J., Verzar, Z., et al., Serotonin may alter the pattern of gonadotropin-induced progesterone release of human granulosa cells in superfusion system, Endocrine, 2004, vol. 24, no. 2, pp. 155–159.
Kranc, W., Budna, J., Kahan, R., et al., Molecular basis of growth, proliferation, and differentiation of mammalian follicular granulosa cells, J. Biol. Regul. Homeost. Agents, 2017, vol. 31, no. 1, pp. 1–8.
Mawe, G.M. and Hoffman, J.M., Serotonin signalling in the gut-functions, dysfunctions and therapeutic targets, Nat. Rev. Gastroenterol. Hepatol., 2013, vol. 10, no. 8, pp. 473–486.
Mercado, C.P. and Kilic, F., Molecular mechanisms of SERT in platelets: regulation of plasma serotonin levels, Mol. Interv., 2010, vol. 10, no. 4, pp. 231–241.
Nikishin, D.A., Alyoshina, N.M., Semenova, M.L., et al., The influence of serotonin on the expression of markers of the functional state of granulosa cells in culture in vitro, Fundam. Aspekty Psikh. Zdor., 2018a, no. 4, pp. 12–17.
Nikishin, D.A., Alyoshina, N.M., and Shmukler, Yu.B., Synthesis and membrane transport of serotonin in the developing ovarian follicle of mouse, Dokl. Biochem. Biophys., 2018b, vol. 478, pp. 4–7.
Nikishin, D.A., Khramova, Yu.V., Bagaeva, T.S., et al., Expression of components of the serotonergic system in folliculogenesis and preimplantation development in mice, Russ. J. Dev. Biol., 2018c, vol. 49, no. 3, pp. 184–192.
Nikishin, D.A., Alyoshina, N.M., Semenova, M.L., et al., Analysis of expression and functional activity of aromatic l-amino acid decarboxylase (DDC) and serotonin transporter (SERT) as potential sources of serotonin in mouse ovary, Int. J. Mol. Sci., 2019, vol. 20, no. 12, p. 3070.
Paulmann, N., Grohmann, M., Voigt, J.-P., et al., Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation, PLoS Biol., 2009, vol. 7, no. 10. e1000229.
Shmukler, Yu.B. and Nikishin, D.A., On the intracellular transmitter reception, Neurochem. J., 2018, vol. 12, no. 4, pp. 295–298.
Walther, D.J., Peter, J.-U., Winter, S., et al., Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release, Cell, 2003, vol. 115, no. 7, pp. 851–862.
Zha, W., Ho, H.T.B., Hu, T., et al., Serotonin transporter deficiency drives estrogen-dependent obesity and glucose intolerance, Sci. Rep., 2017, vol. 7, no. 1, p. 1137.
Zhen, Y.-H., Wang, L., Riaz, H., et al., Knockdown of CEBPβ by RNAi in porcine granulosa cells resulted in S phase cell cycle arrest and decreased progesterone and estradiol synthesis, J. Steroid. Biochem. Mol. Biol., 2014, vol. 143, pp. 90–98.
ACKNOWLEDGMENTS
The work was performed using the equipment of the Core Centrum (Koltsov Institute of Developmental Biology, Russian Academy of Sciences) and the Center for Collective Use (Moscow State University).
Funding
The work was performed within the framework of 2020 State Assignment of Koltzov Institute of Developmental Biology of Russian Academy of Sciences, no. 0108-2019-0003. The study into the effects of fluoxetine was carried out with the financial support of the Russian Science Foundation, project no. 18-74-00143.
Author information
Authors and Affiliations
Contributions
D.A. Nikishin invented and designed the experiments, analyzed the data, and wrote the article. Y.V. Khramova and L.A. Malchenko performed cell culture work and set up experiments. N.M. Alyoshina performed a molecular genetic analysis. Y.B. Shmukler supervised the study and participated in the preparation of the text.
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. In this study, all manipulations performed with experimental animals and methods of anesthesia, euthanasia, and animal care before and after experimental interventions were in accordance with international standards on bioethics.
Additional information
Translated by A. Ermakov
Supplementary Information
Rights and permissions
About this article
Cite this article
Nikishin, D.A., Khramova, Y.V., Alyoshina, N.M. et al. Oocyte-Mediated Effect of Serotonin on the Functional Status of Granulosa Cells. Russ J Dev Biol 52, 97–104 (2021). https://doi.org/10.1134/S1062360421020065
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062360421020065