Abstract
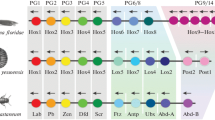
The concept of regeneration is intimately associated with ideas about positional information, that is, the distribution of various signals prescribing cells their location in an embryo or an adult organism. Hox genes are perfect candidates for the role of factors creating positional information. Their main function is thought to be regionalization of the embryo and the determination of the anterior/posterior (A/P) axis of the bilaterian body according to the rules of temporal and spatial colinearity. At the same time, Hox genes are also expressed postembryonically and may participate in various processes in the adult body. In particular, Hox genes are involved in regeneration, as shown on animals from different evolutionary clades. During reparation Hox genes are responsible for regionalization and specification of the newly formed structures, which reflects their embryonic role. This is not all, however. Hox transcription patterns in some adult organisms and their expression dynamics after damage suggest that Hox genes are involved in creating positional information in the adult body. This information is necessary for consistent reparation, while its fast reorganization may accelerate the reparative process.
Similar content being viewed by others
References
Ackema, K.B. and Charité, J., Mesenchymal stem cells from different organs are characterized by distinct topographic Hox codes, Stem Cells Dev., 2008, vol. 17, pp. 979–991.
Agata, K., Tanaka, T., Kobayashi, C., et al., Intercalary regeneration in planarians, Dev. Dynam., 2003, vol. 226, pp. 308–316.
Argiropoulos, B. and Humphries, R.K., Hox genes in hematopoiesis and leukemogenesis, Oncogene, 2007, vol. 26, pp. 6766–6776.
Awgulewitsch, A., Hox in hair growth and development, Naturwissenschaften, 2003, vol. 90, pp. 193–211.
Bakalenko, N.I., Novikova, E.L., Nesterenko, A.Y., et al., Hox gene expression during postlarval development of the polychaete Alitta virens, EvoDevo, 2013, vol. 4, p. 13.
Brun, A.C., Björnsson, J.M., Magnusson, M., et al., Hoxb4-deficient mice undergo normal hematopoietic development but exhibit a mild proliferation defect in hematopoietic stem cells, Blood, 2004, vol. 103, pp. 4126–4133.
Bryant, S.V., Endo, T., and Gardiner, D.M., Vertebrate limb regeneration and the origin of limb stem cells, Int. J. Dev. Biol., 2002, vol. 46, pp. 887–896.
Campbell, G. and Tomlinson, A., Initiation of the proximodistal axis in insect legs, Development, 1995, vol. 121, pp. 619–628.
Carlson, B.M., Principles of Regenerative Biology, Burlington, MA: Academic Press, 2007, pp. 1–24.
Chang, H.Y., Chi, J.T., Dudoit, S., et al., Diversity, topographic differentiation, and positional memory in human fibroblasts, Proc. Natl. Acad. Sci. U. S. A., 2002, vol. 99, pp. 12877–12882.
Cheng, W., Liu, J., Yoshida, H., et al., Lineage infidelity of epithelial ovarian cancers is controlled by HOX genes that specify regional identity in the reproductive tract, Nature Med., 2005, vol. 11, pp. 531–537.
Cho, S.J., Koh, K.S., Lee, E., et al., Differential expression of three labial genes during earthworm head regeneration, Biosci. Biotechnol. Biochem., 2009, vol. 73, pp. 2609–2614.
Chung, N., Jee, B.K., Chae, S.W., et al., HOX gene analysis of endothelial cell differentiation in human bone marrow-derived mesenchymal stem cells, Mol. Biol. Rep., 2009, vol. 36, pp. 227–235.
Endo, T., Bryant, S.V., Gardiner, D.M., A stepwise model system for limb regeneration, Dev. Biol., 2004, vol. 270, pp. 135–145.
Fischbach, N.A., Rozenfeld, S., Shen, W., et al., HOXB6 overexpression in murine bone marrow immortalizes a myelomonocytic precursor in vitro and causes hematopoietic stem cell expansion and acute myeloid leukemia in vivo, Blood, 2005, vol. 105, pp. 1456–1466.
French, V., Leg regeneration in the cockroach, Blatella germanica. II. Regeneration from a non-congruent tibial graft host junction, J. Embryol. Exp. Morphol., 1976, vol. 35, pp. 267–301.
Gardiner, D.M. and Bryant, S.V., Molecular mechanisms in the control of limb regeneration: the role of homeobox genes, Int. J. Dev. Biol., 1996, vol. 40, pp. 797–805.
Han, M., Yang, X., Lee, J., et al., Development and regeneration of the neonatal digit tip in mice, Dev. Biol., 2008, vol. 315, pp. 125–135.
Iten, L.E. and Bryant, S.V., The interaction between the blastema and stump in the establishment of the anterior-posterior and proximal-distal organization of the limb regenerate, Dev. Biol., 1975, vol. 44, pp. 119–147.
Ivanov, P.P., Obshchaya i sravnitel’naya embriologiya (General and Comparative Embryology), Leningrad: Biomedgiz, 1937.
Klausen, C., Leung, P.C., and Auersperg, N., Cell motility and spreading are suppressed by HOXA4 in ovarian cancer cells: possible involvement of beta1 integrin, Mol. Cancer. Res., 2009, vol. 7, pp. 1425–1437.
Ko, K.H., Lam, Q.L., Zhang, M., et al., Hoxb3 deficiency impairs B lymphopoiesis in mouse bone marrow, Exp. Hematol., 2007, vol. 35, pp. 465–475.
Kulakova, M., Bakalenko, N., Novikova, E., et al., Hox gene expression in larval development of the polychaetes Nereis virens and Platynereis dumerilii (Annelida, Lophotrochozoa), Dev. Genes. Evol., 2007, vol. 217, pp. 39–54.
Leucht, P., Kim, J.B., Amasha, R., et al., Embryonic origin and Hox status determine progenitor cell fate during adult bone regeneration, Development, 2008, vol. 135, pp. 2845–2854.
Maden, M., Vitamin a and pattern formation in the regenerating limb, Nature, 1982, vol. 295, pp. 672–675.
Maden, M., The effect of vitamin A (retinoids) on pattern formation implies a uniformity of developmental mechanisms throughout the animal kingdom, Acta Biotheor., 1993, vol. 41, no. 4, pp. 425–445.
Mahdipour, E. and Mace, K.A., Hox transcription factor regulation of adult bone-marrow-derived cell behaviour during tissue repair and regeneration, Expert Opin. Biol. Ther., 2011, vol. 11, pp. 1079–1090.
Mahdipour, E., Charnock, J.C., and Mace, K.A., Hoxa3 promotes the differentiation of hematopoietic progenitor cells into proangiogenic Gr-1+ CD11b+ myeloid cells, Blood, 2011, vol. 117, pp. 815–826.
Mark, M., Rijli, F.M., and Chambon, P., Homeobox genes in embryogenesis and pathogenesis, Pediatr. Res., 1997, vol. 42, pp. 421–429.
Martin, P., Wound healing-aiming for perfect skin regeneration, Science, 1997, vol. 276, pp. 75–81.
Mohanty-Hejmadi, P., Dutta, S.K., and Mahapatra, P., Limbs generated at site of tail amputation in marbled balloon frog after vitamin a treatment, Nature (London), 1992, vol. 355, pp. 352–353.
Morgan, R. and Whiting, K., Differential expression of HOX genes upon activation of leukocyte sub-populations, Int. J. Hematol., 2008, vol. 87, pp. 246–249.
Nachtrab, G., Kikuchi, K., Tornini, V.A., et al., Transcriptional components of anteroposterior positional information during zebrafish fin regeneration, Development, 2013, vol. 140, no. 18, pp. 3754–3764.
Niazi, I.A. and Saxena, S., Abnormal hind limb regeneration in tadpoles of the toad, Bufo andersoni, exposed to excess vitamin, Folia Biol. (Krakow), 1978, vol. 26, pp. 3–8.
Nicolas, S., Papillon, D., Perez, Y., et al., The spatial restrictions of 5' HoxC genes expression are maintained in adult newt spinal cord, Biol. Cell, 2003, vol. 95, pp. 589–594.
Niederreither, K. and Dolle, P., Retinoic acid in development: towards an integrated view, Nat. Rev. Genet., 2008, vol. 9, pp. 541–553.
Nogi, T. and Watanabe, K., Position-specific and noncolinear expression of the planarian posterior (Abdominal-B-like) gene, Dev. Growth Differ., 2001, vol. 43, pp. 117–184.
Novikova, E.L., Bakalenko, N.I., Nesterenko, A.Y., et al., Expression of Hox genes during regeneration of nereid polychaete Alitta (Nereis) virens (Annelida, Lophotrochozoa), EvoDevo, 2013, vol. 4, p. 14.
Okada, T.S.J., A brief history of regeneration research—for admiring Professor Niazi’s discovery of the effect of vitamin A on regeneration, J. Biosci., 1996, vol. 21, pp. 261–271.
Orii, H., Kato, K., Umesono, Y., et al., The planarian Hom/Hox homeobox genes (Plox) expressed along the anteroposterior axis, Dev. Biol., 1999, vol. 210, pp. 456–468.
Pfeifer, K., Dorresteijn, A.W., and Frobius, A.C., Activation of Hox genes during caudal regeneration of the polychaete annelid Platynereis dumerilii, Dev. Genes Evol., 2012, vol. 222, pp. 165–179.
Savard, P., Gates, P.B., and Brockes, J.P., Position dependent expression of a homeobox gene transcript in relation to amphibian limb regeneration, EMBO J., 1988, vol. 7, pp. 4275–4282.
Shah, N. and Sukumar, S., The Hox genes and their roles in oncogenesis, Nat. Rev. Cancer, 2010, vol. 10, pp. 361–371.
Simon, H.G. and Tabin, C.J., Analysis of Hox-4.5 and Hox-3.6 expression during newt limb regeneration: differential regulation of paralogous Hox genes suggest different roles for members of different Hox clusters, Development, 1993, vol. 117, pp. 1397–1407.
Slack, J.M.W., Morphogenetic gradients-past and present, Trends Biol. Sci., 1987, vol. 12, pp. 200–204.
Thummel, R., Ju, M., Sarras, M.P., et al., Both Hoxc13 orthologs are functionally important for zebrafish tail fin regeneration, Dev. Genes Evol., 2007, vol. 217, pp. 413–420.
Yang, M., Li, Q.F., and Zhang, F., Hox genes in the skin, Chin. Med. J. (Engl.), 2010, vol. 123, pp. 2607–2612.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Ontogenez, 2016, Vol. 47, No. 4, pp. 209–218.
The article was translated by the authors.
Rights and permissions
About this article
Cite this article
Novikova, E.L., Bakalenko, N.I., Nesterenko, A.Y. et al. Hox genes and animal regeneration. Russ J Dev Biol 47, 173–180 (2016). https://doi.org/10.1134/S106236041604007X
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S106236041604007X