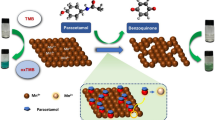
Abstract
We proposed a voltammetric DNA sensor based on glassy carbon modified with carbon black, pillar[5]arene, and electropolymerized Neutral Red. To increase signal sensitivity, Methylene Blue in monomeric and polymeric forms was introduced into the surface layer of the DNA sensor as a specific DNA intercalator and electron transfer mediator. The electrochemical characteristics of the coating are determined, depending on the composition and the preparation method; a consistent change in the peak currents of dyes is observed during the incubation of a DNA sensor in a solution of doxorubicin as a model anticancer drug. Under optimal conditions, the DNA sensor enables the determination of 10 nM to 0.1 mM of doxorubicin. Doxorubicin can also be determined in synthetic blood plasma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The creation of sensitive devices capable of detecting the presence of low-molecular-weight compounds that damage or specifically interact with DNA is an urgent trend in the development of modern analytical chemistry. Such devices include sensors for screening new preparations, including anticancer drugs, and for monitoring food contamination. At present, high-performance liquid chromatography and capillary electrophoresis are mainly used for these purposes [1, 2]. However, these methods have several disadvantages, for example, the requirement for highly skilled operators, laborious sample preparation in a stationary laboratory, and the high cost of equipment. Biosensors, miniature analytical devices, provide an affordable alternative to universal analytical equipment [3]. Biosensors containing DNA as an element of biorecognition are especially attractive, because the biological receptor acts as a model of recorded biochemical interactions in a human body. DNA can interact with biologically active molecules with high specificity due to hydrogen, electrostatic, and donor–acceptor bonds and hydrophobic effects [4]. In addition to detecting hybridization of complementary DNA sequences in the diagnosis of pathogenic microorganisms and viruses, DNA sensors are actively used to analyze anticancer drugs and determine reactive oxygen species [5].
Doxorubicin, an anthracycline drug whose cytostatic mechanism of action is based on intercalation into the DNA double helix, is often used as a model analyte to develop such DNA sensors [4]. Many works have been presented on determining doxorubicin using various DNA sensors, primarily electrochemical ones [6–8]. However, improving the design, simplifying the signal measurement protocol, and enhancing the analytical performance of the DNA sensor remain challenges. To solve them, we need to improve the immobilization of DNA in the biosensor and optimize the conditions for signal recording.
Modifying the electrode surface with electrochemically active components decreases the working potential of the biosensor and improves its analytical characteristics. One of promising methods of such modification is the deposition of electrochemically active polymer films exhibiting mediator properties [9]. An example is provided by a biosensor with a surface layer of polyaniline and DNA [10]. However, the electropolymerization of aniline is carried out exclusively in strong mineral acids. It exhibits electrical conductivity and electrochemical activity in acidic media, which complicates the incorporation of DNA and the subsequent use of the biosensor to analyze real samples. An alternative is the use of polymeric forms of phenazine and phenothiazine dyes. Their polymerization occurs in the pH range 6–8, and the electropolymerized form exhibits reversibility of electron transfer in a wide range of signal measurement conditions [11]. The interaction of phenazines and phenothiazines with DNA does not block the electrochemical activity of these compounds completely, which enables them to retain the ability to transfer an electron [5].
We have previously proposed a DNA sensor based on carbon black (CB), pillar[5]arene (P5A), and poly(Neutral Red) (polyNR) for recording oxidative damage to DNA and measuring the antioxidant activity of compounds [12]. Neutral Red is a phenazine dye that can polymerize upon multiple cycling of the potential with the formation of an electroactive polymer [13]. Poly(Neutral Red) has found application in various sensors and biosensors as a mediator of electron transfer [14–17]. At the same time, the reaction of polyNR to biochemical reactions involving DNA remains insufficiently studied; moreover, the reagent demonstrates low selectivity and low sensitivity towards low-molecular-weight analytes. These characteristics can be improved by optimizing the conditions for introducing DNA into the composition of the surface layer of the biosensor, for example, by including the biopolymer into the dye monomer solution at the stage of electropolymerization [6]. The scanning electron microscopy data showed that in this case, the copolymer film was obtained denser, stronger, and rougher than the films of the dye in the absence of DNA. The introduction of DNA improved the sensitivity of the determination of doxorubicin with impedimetric signal recording. At the same time, the sensitivity of the voltammetric recording of the response remained insufficient.
In this work, we have proposed a hybrid coating of an electrochemical DNA sensor, which includes carbon black, pillar[5]arene, and poly(Neutral Red) with the addition of an electron transfer mediator, Methylene Blue (MB), to the layer. Methylene Blue has high electrochemical activity and actively interacts with DNA by the mechanism of intercalation and electrostatic adsorption [18, 19]. This enables the use of the competitive interaction of Methylene Blue and doxorubicin for the highly sensitive determination of the latter. We considered both the preparation of a dye copolymer and the introduction of the monomeric form of Methylene Blue into a buffer solution for recording cyclic voltammograms (CVAs) as an additional factor that increases the sensitivity for recording biospecific interactions with the participation of DNA.
EXPERIMENTAL
Reagents. We used DNA from fish milk, Neutral Red (n8,n8,3-trimethylphenazine-2,8-diamine chloride), Methylene Blue (3,7-bisdimethylaminophenothiazine chloride), chitosan (Sigma-Aldrich), and carbon black (IMERYS, Belgium). Pillar[5]arene was synthesized at the Department of Organic Chemistry, Kazan Federal University, according to the method [20]. Its chemical structure was confirmed by IR spectroscopy, 1H NMR spectroscopy, and elemental analysis.
All other reagents were of analytical grade and were used without further purification. Solutions for electrochemical measurements were prepared in water deionized using a Millipore® setup. To control the pH of the working solutions, we used an Expert-001 pH meter-potentiometer (Econix-Expert, Russia).
Voltammetric measurements were performed using a CHI 660E electrochemical analyzer (CH Instruments, United States) in a three-electrode cell. It included a glassy carbon electrode (GCE) made of a SU2000 rod with a diameter of 2 mm (NIIGrafit, Moscow) in a Teflon casing equipped with a steel current collector. The counter electrode was a platinum wire electrode (CHI 129, CH Instruments, United States), and the reference electrode was an Ag/AgCl (1 M KCl) electrode (CHI 128, CH Instruments, United States).
Preliminary modification of the electrode with carbon black and pillar[5]arene. The glassy carbon electrode was polished, rinsed in ethanol, and then purified by cycling its potential in the range from –1 to 1 V in 0.2 M sulfuric acid until the currents were stabilized. Then, 1 µL of carbon black (1.3 mg/mL in a 0.375% solution of chitosan in 0.05 M HCl) and 0.8 µL of 1 M NaOH solution were applied to the GCE surface twice, each layer was dried at 60°C, according to the procedure [12]. Two microliters of a 1 × 10–4 M solution of pillar[5]arene in acetone was applied over carbon black and dried in air. The resulting modified electrode was covered with a film of polyNR, polyMB/polyNR, or a copolymer of NR and MB. The electropolymerization of Neutral Red was carried out by cycling the potential in the range from –0.8 to 0.8 V in a phosphate buffer solution containing 25 mM of NaH2PO4, 0.1 M of NaNO3 (pH 6.0), and 0.4 mM of Neutral Red. The electropolymerization of Methylene Blue was carried out by cycling the potential in the range from –0.65 to 1.2 V in a phosphate buffer solution containing 25 mM of NaH2PO4, 100 M of Na2SO4 (pH 8.2), and 10 mM of Methylene Blue. After the electropolymerization, the electrode was transferred to a working buffer solution containing no monomer, and ten potential scan cycles were recorded to remove unbound dye particles from the polymer film. DNA of various concentrations (0.1, 0.2, 0.3, 0.5, 1.0, and 2.0 mg/mL) was included in the growing polymer film at the stage of dye electropolymerization.
Determination of the doxorubicin concentration. The DNA sensor was incubated in a doxorubicin solution in water or synthetic blood plasma. The analytical signal was the change in the redox current peaks of the polymeric forms of the dyes, recorded in a 25 mM phosphate buffer solution containing 0.1 M Na2SO4 (pH 5.0). Artificial blood plasma contained 4 × 10–6 M of L-methionine, 2 × 10–3 M of NaCl, 2 × 10–4 M of NaHCO3, 1.3 × 10–6 M of L-cysteine, 3.5 × 10–6 M of L-glycine, 2.1 × 10–4 M of L-tryptophan, 2 × 10–4 M of L-tyrosine, 4 × 10–6 M of L-phenylalanine, 5 × 10–6 M of D,L-lysine, 3.5 × 10–6 M of L-histidine, 2.2 × 10–5 M of L-aspartic acid, 5 × 10–6 M of L-arginine, and 2 × 10–4 M of L-alanine [21].
RESULTS AND DISCUSSION
Measurement of poly(Neutral Red) signal in the presence of monomeric Methylene Blue and DNA. To confirm a possibility of joint use of two dyes in the composition of a DNA sensor, we first examined the poly(Neutral Red) films obtained in the absence and presence of DNA, followed by the introduction of monomeric Methylene Blue into the working buffer solution. The corresponding CVAs are shown in Fig. 1. Measurements were carried out at pH 5.0, corresponding to the best reproducibility of peaks in voltammograms [5, 12].
Cyclic voltammograms obtained using glassy carbon electrodes coated with (a) CB/P5A/polyNR and (b) CB/P5A/(polyNK + DNA) in a 25 mM phosphate buffer solution with pH 5.0 containing 0.1 M of Na2SO4 and Methylene Blue in various concentrations: (0) 0, (1) 1 × 10–6, (2) 5 × 10–6, (3) 1 × 10–5, (4) 5 × 10–5, and (5) 1 × 10–4 M.
In the presence of Methylene Blue, two pairs of peaks were observed for the modified electrode. The peaks in the potential range from –0.4 to –0.6 V corresponded to poly(Neutral Red), and the peaks in the range from –0.2 to –0.1 V belonged to monomeric Methylene Blue. As the concentration of Methylene Blue in the solution was increased, the anodic peaks of the poly(Neutral Red) decreased in magnitude, remaining stable in successive measurements on the same electrode. At the same time, the cathodic peaks of poly(Neutral Red) decreased almost twofold compared to the values obtained in the absence of Methylene Blue. This indicates the reduced form of Methylene Blue in the electron transfer chain with the participation of the oxidized form of poly(Neutral Red). The Methylene Blue peak currents showed a slight decrease when the dye was introduced into the coating with DNA. Probably, some of the dye molecules intercalated into the DNA double helix, partially losing the ability to oxidize/reduce at the electrode.
The currents of the poly(Neutral Red) peak after measurement in a Methylene Blue solution also decreased after moving the modified electrode into a buffer solution that did not contain Methylene Blue. The poly(Neutral Red) currents remained at the same level, while the Methylene Blue currents, although significantly decreased, remained quite pronounced. This is due to the penetration of Methylene Blue into the composition of the modifying coating and its retention in the layer during measurements in a solution that does not contain Methylene Blue. The accumulation of Methylene Blue in the surface layer was also confirmed by an experiment with a preliminary 10-min keeping of the modified electrode in a solution of Methylene Blue of various concentrations. The currents of the oxidation peak of poly(Neutral Red) continued to be more stable than the currents of the reduction peak. For lower concentrations of Methylene Blue, its currents, especially reduction currents, in the case of initial exposure, significantly increased relative to the values obtained without such incubation. This is due to the electrostatic concentration of positively charged Methylene Blue molecules in the coating composition containing DNA molecules with negatively charged phosphate groups of the backbone.
The optimal concentration of pillar[5]arene, a macrocyclic mediator ща electron transfer, was determined by the results of experiments with varying its concentration at the stage of applying to the surface of a GCE modified with carbon black. The use of mediator solutions in the concentration range of 1 × 10–7–5 × 10–5 M does not lead to any significant changes in the electrochemical behavior of the redox-active poly(Neutral Red) polymer. An increase in the concentration of pillar[5]arene in an aliquot to 0.1 mM significantly improves the conditions of electron exchange within the framework of the modifying coating, resulting in a change in the morphology and magnitude of the poly(Neutral Red) peaks, which became more pronounced. Using the indicated concentration of pillar[5]arene also had a positive effect on the peaks of diffusion-free Methylene Blue. However, a further increase in the pillar[5]arene concentration led to a deterioration in the response of the modifying coating due to the probable aggregation of macrocycle molecules and blocking of the surface available for further electrodeposition of poly(Neutral Red). In this regard, a pillar [5]arene concentration of 0.1 mM was taken as the optimal one and used in further experiments.
Varying the DNA concentration in the range of 0.1–0.3 mg/mL in the solution for electropolymerization demonstrated the absence of significant differences in the dye signals. In using DNA concentrations of 0.5 to 2.0 mg/mL, the dye currents decreased due to the partial blocking of the sensor surface by nonconducting DNA molecules. The highest stability of the dye signals in a series of sequential measurements was shown by the coating containing 0.2 mg/mL of DNA; therefore, this value of the biopolymer concentration was used in further experiments.
Copolymerization of Neutral Red and Methylene Blue. Although the monomeric form of Methylene Blue is beneficial for the redox properties of the CB/P5A/polyNR hybrid coating, the service life and mechanical stability of such a biosensor are limited by desorption of the Methylene Blue monomer from the electrode surface. Electropolymerization of Methylene Blue could improve the stability of the coating.
We considered options of assembling the surface layer of a biosensor by copolymerizing dyes from a common solution of monomers and by sequential electropolymerization of Neutral Red followed by Methylene Blue. The corresponding CVAs are shown in Fig. 2. During electropolymerization from a solution of Neutral Red and Methylene Blue, the redox peaks of poly(Neutral Red) are weakly expressed, and the successive electropolymerization (electrodeposition of poly(Methylene Blue) over poly(Neutral Red)), only the peaks of poly(Methylene Blue) were observed in the CVA, apparently due to its higher efficiency in the reaction of electron transfer.
For coatings with different compositions of the polymeric layer based on poly(Neutral Red), we studied the dependences of the current and potential of the electroconversion peaks of the polymeric forms of Neutral Red and Methylene Blue (Table 1). Interestingly, the slope of the dependence of the peak current on the scan rate of the potential in bilogarithmic coordinates remains at a level of 0.5–0.6, formally corresponding to the diffusion control of electron transfer, although there are no diffusion-free dyes in the solution. This is due to the limitation of the overall reaction rate by electron exchange processes between oxidized and reduced forms of dyes in the surface layer. The only exception can be the currents of the poly(Methylene Blue) peak, which are significantly higher than the poly(Neutral Red) peaks, regardless of the electropolymerization protocol, and approach the value typical for limiting the process by surface stages. This is consistent with the ratio of the reactivity of the polymeric forms of dyes, determined based on the change in the peak currents of mixed coatings relative to the properties of the poly(Neutral Red) film. Another remarkable result is an almost complete absence of the pH dependence of the potentials of the poly(Neutral Red) peak. We can assume that the stages of protonation/deprotonation of this dye do not affect the process rate due to the coordinated transfer of hydrogen ions and electrons to the redox centers of Methylene Blue present in the layer.
Determination of doxorubicin. To determine doxorubicin using the developed DNA sensor, CVAs were recorded before and after incubation of the DNA sensor in the analyte solution (Fig. 3). The supposed increase in the sensitivity of the biosensor is associated with competitive interactions between Methylene Blue and doxorubicin; therefore, a biosensor with monomeric Methylene Blue in the layer was used in these experiments.
Cyclic voltammograms recorded using glassy carbon electrodes modified with CB/P5A/(polyNR + DNA) in a 25 mM phosphate buffer solution with pH 5.0, containing 0.1 M of Na2SO4, (a) in the absence and (b) the presence of 1 × 10–4 M of Methylene Blue after incubation in an aqueous solution of doxorubicin with a concentration of (1) 1 × 10–8, (2) 1 × 10–7, (3) 1 × 10–6, (4) 1 × 10–5, and (5) 1 × 10–4 M.
With an increase in the concentration of doxorubicin, the Methylene Blue peak currents increased, which is associated with the competitive displacement of the dye from DNA molecules and an increase in its reactivity in the redox reaction at the electrode. The poly(Neutral Red) peak currents showed a similar tendency due to an increase in the efficiency of electron exchange in the layer with an increase in the fraction of free Methylene Blue molecules. All measurements were performed once since the removal of doxorubicin from the surface layer of the biosensor was accompanied by partial destruction of the coating or disturbance of the layer structure and a sharp decrease in the currents recorded in the CVA. Under optimal conditions for measuring the signal, the biosensor enables the determination of doxorubicin in the concentration range of 10 nM to 0.1 mM with a detection limit of 3 nM (the calibration equation is log (I, µA) = (2.85 ± 0.01) + (0.142 ± 0.002)log (c, M), n = 5, R2 = 0.9991). For comparison, the sensitivity of the determination of doxorubicin by the anodic current of the poly(Neutral Red) peak in the absence of Methylene Blue was 0.09 µA/pc. The found characteristics of the determination of doxorubicin are comparable or better than the parameters of electrochemical sensors with the voltammetric signal recording (Table 2). The biosensor retains the signal for doxorubicin when stored before measurement for at least 30 days (in the absence of moisture at 4°C).
Similar measurements were performed using synthetic blood plasma. In the absence of Methylene Blue, the sensitivity of the poly(Neutral Red) signal to doxorubicin is comparable to that found earlier for standard solutions of the analyte when measured in a buffer solution (0.07 ± 0.01). In synthetic plasma, the slope of the corresponding dependence is slightly higher (the calibration equation is log (I, µA) = (3.76 ± 0.02) + (0.215 ± 0.004)log (c, M), n = 5, R2 = 0.9986). This may be due to the effect of synthetic plasma components on the adsorption retention of Methylene Blue and its signal. Nevertheless, when the plasma is diluted tenfold with the working buffer solution, this effect disappears. The accuracy of the determination of doxorubicin in 1 : 10 diluted plasma is given in Table 3.
Cyclic voltammograms recorded using a glassy carbon electrode modified with CB/P5A/(polyNR + DNA) in a 25 mM phosphate buffer solution with pH 5.0, containing 0.1 M of Na2SO4, (a) in the absence and (b) the presence of 1 × 10–4 M of Methylene Blue after incubation in a solution of synthetic blood plasma containing doxorubicin at a concentration of (1) 1 × 10–8, (2) 1 × 10–7, (3) 1 × 10–6, (4) 1 × 10–5, and (5) 1 × 10–4 M.
Change history
31 March 2022
An Erratum to this paper has been published: https://doi.org/10.1134/S1061934822330012
REFERENCES
Kozlu, S., Sahin, A., Calis, S., and Capan, Y., Pharmazie, 2017, vol. 72, no. 10, p. 568. https://doi.org/10.1691/ph.2017.7077
Robieux, I., Aita, P., Sorio, R., Toffoli, G., and Boiocchi, M., J. Chromatogr. B: Biomed. Sci. Appl., 1996, vol. 685, p. 383. https://doi.org/10.1016/s0378-4347(96)00300-3
Ribeiro, D.B., Silva, G.S., dos Santos, D.R., Costa, A.R., Ribeiro, E.B., Badea, M., and Nunes, G.S., Antioxidants, 2021, vol. 10, no. 2, p. 324. https://doi.org/10.3390/antiox10020324
Evtyugin, G.A., Budnikov, G.K., and Porfir’eva, A.V., Russ. J. Gen. Chem., 2008, vol. 78, no. 12, p. 2489. https://doi.org/10.1134/S107036320812030X
Kuzin, Y., Porfireva, A., Stepanova, V., Evtugyn, V., Stoikov, I., Evtugyn, G., and Hianik, T., Electroanalysis, 2015, vol. 27, no. 12, p. 2800. https://doi.org/10.1002/elan.201500312
Porfireva, A. and Evtugyn, G., Nanomaterials, 2020, vol. 10, no. 5, p. 924. https://doi.org/10.3390/nano10050924
Asai, K., Yamamoto, T., Nagashima, S., Ogata, G., Hibino, H., and Eynaga, Y., Analyst, 2020, vol. 145, no. 2, p. 544. https://doi.org/10.1039/C9AN01976F
Hajian, R., Tayebi, Z., and Shams, N., J. Pharm. Anal., 2017, vol. 7, no. 1, p. 27. https://doi.org/10.1016/j.jpha.2016.07.005
Yang, R., Ruan, C., and Deng, J., J. Appl. Electrochem., 1998, vol. 28, no. 11, p. 1269. https://doi.org/10.1023/A:1003460431109
Kulikova, T.N., Porfireva, A.V., Shamagsumova, R.V., and Evtugyn, G.A., Electroanalysis, 2018, vol. 30, p. 1. https://doi.org/10.1002/elan.201800331
Karyakin, A.A., Karyakina, E.E., and Shmidt, H.-L., Electroanalysis, 1999, vol. 11, no. 3, p. 149. https://doi.org/10.1002/(SICI)1521-4109(199903)11:3<149::AID-ELAN149>3.0.CO;2-G
Kuzin, Y., Kappo, D., Porfireva, A., Shurpik, D., Stoikov, I., Evtugyn, G., and Hianik, T., Sensors, 2018, vol. 18, no. 10, p. 3489. https://doi.org/10.3390/s18103489
Benito, D., Gabrielli, C., Garcia-Jareno, J.J., Keddam, M., Perrot, H., and Vicente, F., Electrochim. Acta, 2003, vol. 48, p. 4039. https://doi.org/10.1016/S0013-4686(03)00561-9
Sunil Kumar Naik, T.S. and Swamy, K., Electroanal. Chem., 2017, vol. 804, p. 78. https://doi.org/10.1016/j.jelechem.2017.08.047
Romero, P.R., Gonzalez-Rodriguez, J., Rodriguez-Amaro, R., and Rodriguez Mellado, J.M., Electrochim. Acta, 2018, vol. 48, p. 4039. https://doi.org/10.1016/j.electacta.2018.03.135
Devi, C.L. and Narayanan, S.S., Bull. Mater. Sci., 2019, vol. 42, p. 73. https://doi.org/10.1007/s12034-019-1775-7
Pauliukaite, R., Ghica, M.E., and Barsan, M., J. Solid State Electrochem., 2007, vol. 11, p. 899. https://doi.org/10.1007/s10008-007-0281-9
Vanickova, M., Buckova, M., and Labuda, J., Chem. Anal., 2000, vol. 45, p. 125.
Zhong, J., Qi, Z., Dai, H., Fan, C., Li, G., and Matsuda, N., Anal. Sci., 2003, vol. 19, p. 653. https://doi.org/10.2116/analsci.19.653
Ogoshi, T., Aoki, T., Kitajima, K., Fujinami, S., Yamagishi, T.-A., and Nakamoto, Y., Org. Chem., 2011, vol. 76, p. 328. https://doi.org/10.1021/jo1020823
Parham, H. and Zargar, B., Talanta, 2001, vol. 55, p. 255. https://doi.org/10.1016/S0039-9140(01)00416-7
Alizadeh, P.M., Hasanzadeh, M., Soleymani, J., Gharamaleki, J.V., and Jouyban, A., Microchem. J., 2019, vol. 145, p. 450. https://doi.org/10.1016/j.microc.2018.11.012
Ehsani, M., Soleymani, J., Mohammadalizadeh, P., Hasanzadeh, M., Jouyban, A., Khoubnasabjafari, M., and Vaez-Gharamaleki, YMicrochem. J., 2021, vol. 165, 106101. https://doi.org/10.1016/j.microc.2021.106101
Hasanzadeh, M., Hashemzadeh, N., Shadjou, N., Eivazi-Ziaei, J., Knoubnasabjafari, M., and Jouyban, A., J. Mol. Liq., 2016, vol. 221, p. 354. https://doi.org/10.1016/j.molliq.2016.05.082
Ehsani, M., Soleymani, J., Hasanzadeh, M., Vaez-Gharamaleki, Y., Khoubnasabjafaru, M., and Jouyban, A., J. Pharm. Biomed. Anal., 2021, vol. 192, 113701. https://doi.org/10.1016/j.jpba.2020.113701
Materon, E.M., Wong, A., Fatibello-Filho, O., and Faria, R.C., J. Electroanal. Chem., 2018, vol. 827, p. 64. https://doi.org/10.1016/j.jelechem.2018.09.010
Deepa, S., Kumara Swamy, B.E., and Vasantakuma Pai, K., J. Electroanal. Chem., 2020, vol. 879, 114748. https://doi.org/10.1016/j.jelechem.2020.114748
Kalambate, P.K., Li, Y., Shen, Y., and Huang, Y., Anal. Methods, 2019, vol. 11, p. 443. https://doi.org/10.1039/C8AY02381F
Er, E. and Erk, N., Microchim. Acta, 2020, vol. 187, p. 223. https://doi.org/10.1007/s00604-020-4206-y
Funding
This work was supported by the Russian Foundation for Basic Research, project no. 20-33-90132.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Zhukova
The original online version of this article was revised: Due to a retrospective Open Access order.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kappo, D., Kuzin, Y.I., Shurpik, D.N. et al. Voltammetric DNA Sensor Based on Redox-Active Dyes for Determining Doxorubicin. J Anal Chem 77, 94–100 (2022). https://doi.org/10.1134/S1061934822010075
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934822010075