Abstract
Using the original high-gas pressure unit (IHPV), experiments were conducted for the first time on the interaction of iron with methane at a temperature of 900°C and a pressure of 100 MPa. Complex methods (microprobe, Raman spectroscopy, chromatography, mass balance calculations) are used for a thorough analysis of fluid compositions and metallic phases formed in experiments. For the first time, experimental and theoretical quantitative data on the composition of the fluid and the composition of the fluid components dissolved in the metal were obtained. Unlike the previously studied Fe3C–H2 system, in experiments, when Fe reacts with methane, there is an active interaction of carbon formed due to the pyrolysis of methane with iron up to the synthesis of Fe3C carbide. The experiments have shown that increasing pressure inhibits significantly hydrogen yield during methane conversion on metallic iron. Carbon saturation of iron with the formation of Fe3C is not complete within the entire volume of the metal during 24 h runs at 900°С. Employing molybdenum containers facilitates CH4 decomposition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 INTRODUCTION
The role of hydrogen, the most abundant element in our galaxy, in natural processes is extremely diverse and in recent years has attracted increasing attention from geochemists and petrologists. The origin of the Earth and its structure, the formation of the Earth’s core, the nucleation of magmas at different levels of depth in different geological epochs and their evolution in the lithosphere, the mechanisms of earthquakes, the origin of the oceans and the Earth’s atmosphere, the degassing of the Earth’s interior, ore formation and the formation of native metals in the earth’s crust are increasingly associated with the active participation of hydrogen in them (for example, [1–4], etc.). And the problem of the possibility of the joint entry of hydrogen and carbon into metallic iron at high and ultrahigh pressures remains debatable [5–7]. Recently, we have obtained new experimental data on the interaction of iron carbide Fe3C with pure hydrogen in the temperature range T = 1273–1423 K and pressures P = 30–100 MPa [8]. The products of the experiments consisted of a metal with a very low carbon content (≤0.3 at %) and a fluid phase enriched with methane (CH4/(CH4 + H2) to 0.37). Raman spectroscopy revealed peaks of hydrogen and disordered carbon in a metal product in one of the experiments, indicating the possibility for both elements to enter the metal phase [8]. It should be noted that the process of carburizing iron using H2/CH4 gas is an effective method for producing iron carbide and has been well studied in relation to metallurgical processes. Carburization of metallic iron begins with the decomposition of methane, and precipitated carbon is the carbon source of iron carbide. It has also been established that the analytical decomposition of methane, using catalysts based on Fe, Ni, Co, Mo alloys deposited on a SiO2 or Al2O3 substrate, is an economical technology, since it makes it possible to obtain both high-purity hydrogen and some nanocarbon materials ([9], etc.).
The proposed work presents the first results of experiments on the interaction of Fe with methane in open sapphire and molybdenum ampoules at a pressure of 100 MPa created by pure CH4, a temperature of 900°C and the duration of kinetic experiments of 16–24 hours.
2 EQUIPMENT AND TECHNIQUES
The experiments were carried out on a unique high gas pressure unit (IHPV). This device is equipped with an original internal device, which made it possible to carry out long-term tests at high temperatures, despite the high penetrating power of hydrogen, formed in experiments due to methane pyrolysis. This device is considered in detail in ([8], Fig. 1), here we will explain in a brief form. The device includes a molybdenum reactor, with molybdenum and sapphire ampoules placed in it with initial Fe samples (approximately 200 mg in each ampoule). The reactor is hermetically connected to a piston equalizer – separator. The internal volumes of the molybdenum reactor and the equalizer–separator under the piston were filled with methane at a pressure of 10 MPa using a special system. The device assembled in this way, together with an internal heater, was placed inside a high-pressure vessel (IHPV), so that the ampoules with the samples are in the gradient-free temperature zone of the heater. Due to the movement of the equalizer-separator piston, the methane pressure in the internal volume of the molybdenum reactor always was kept equal to the gas pressure (Ar) in the vessel (IHPV) during the experiment. At the beginning of the experiment, the pressure of argon in the vessel and, accordingly, methane in the reactor was raised for one hour to the required value of 100 MPa. Next, the temperature of the experiment was raised to the required value of 900°C. At these parameters, the samples were kept in automatic mode for the required time of the experiments (16 or 24 hours), after which it was carried out isobaric quenching with the internal heater of the unit turned off. The rate of quenching of the samples was approximately 300°C/min. The error of measuring the temperature of the experiment was ±5°C, and the pressure of methane ±0.1% rel. After isobaric quenching, pressure relief in the vessel and complete cooling, the internal device was removed from the high-pressure gas pressure vessel (IHPV), fluid samples were taken, and then ampoules with samples were extracted from the molybdenum reactor for subsequent analysis of the phases formed during the experiment. As an initial sample, pieces of chemically pure Fe were used. The chemical composition of the phases obtained in the experiments (Table 1, Fig. 2) was determined using a digital electron X-ray microscope CamScan MV 2300 (VEGA TS 5130 MM), with an attachment for energy-dispersive microanalysis INCA Energy 450 and WDS Oxford INCA Wave 700. The analyses were carried out at an accelerating voltage of 20 kV with a beam current of up to 400 nA and the time of the spectra set is 50–100 s. The carbon content in quenched samples after experiments (see Table 1, Fig. 2) determined by the difference between the sums of the analyzed elements and stoichiometric. This content of C in the samples was additionally controlled by the analysis of the results of the masses—the balance of experiments. Raman spectroscopy is used to confirm the presence of dissolved hydrogen in quenched samples. Raman spectra were obtained on an RM1000 spectrometer (Renishaw) equipped with an SSD camera, an edge filter and a Leica DMLM microscope. The chromatograph “Crystallux–4000M” (GEOKHI RAS) was used to quantify the compositions of the fluid and the components of the fluid dissolved in the samples after experiments (analyst S.G. Naimushin). The chromatographic analysis technique was briefly as follows. With the help of a special device, fluid samples were taken from the internal device of the high-pressure vessel into ampoules with a salt seal (5 pieces), from which fluid samples (500 ml each) were sequentially injected into the gas system of the chromatograph for analysis. Thus, after each experiment, five chromatographic analyses of the fluid composition were carried out to obtain an average value. To determine the composition of the gas dissolved in the samples during the experiments, the solid sample was removed from the ampoule and placed in a special heater built into the gas system of the chromatograph, which had a special valve designed to inlet the releasing gas into the chromatograph system. After the final heating of the sample in this heater (800°C) and holding at this temperature, equal to about 3 minutes, opened the valve and carried out the release of the released gas into the chromatograph system for a fivefold analysis of the composition.
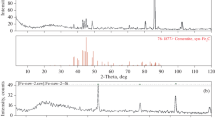
Raman spectrum of the sample (experiment no. 2153) in the region of valence oscillation of hydrogen [9].
Raster micrographs in backscattered electrons (BSE) after quenching Fe samples under methane pressure, experiment no. 2153, (a) sapphire ampoule, (b) molybdenum ampoule. Points 1–7 in Figs. 2a, 2b show analyses, indicated in Figs. 3a, 3c.
3 RESULTS AND DISCUSSION
Figure 1 shows the Raman spectrum obtained on sample from experiment a no. 2153, T = 900°C, P(CH4) = 100 MPa, experiment time 24 h. The peaks in the region of valence oscillations of hydrogen (4150–4215 cm–1) in this sample clearly indicate the presence of dissolved molecular hydrogen. Quantitatively, the concentrations of hydrogen and methane dissolved in the Fe sample, as well as the composition of the fluid that was formed in the experiment (initially chemically pure methane), were determined using chromatographic analysis using the methods briefly described in the previous section. The results are presented in Table 1 as averages from five measurements.
The amount of hydrogen extracted from the inclusions of native iron in gabbro-dolerites of Mount Ozernaya of the Dzhaltul trap intrusive of the Siberian platform was 1.1 cm3/g or 75% of the total composition of the extracted gases ([12], Table 4.3).
In contrast to the previously studied Fe3C–H2 system [8], in the presented experiments there is a pyrolysis of methane and an active interaction of the resulting carbon with Fe up to the synthesis of Fe3C carbide in ampoules on the surface of the samples (Fig. 3). The pyrolysis of methane at the parameters of the experiments is the large amount of soot found in the ampoules with samples after the experiments. Such a significant degree of methane pyrolysis in the experiments is probably due to the catalytic effect of the Fe samples themselves, as well as the reactor material and ampoules (see above). After the experiment, each sample was extracted from the ampoules, cut across and prepared for subsequent microprobe analysis of carbon content profiles (Fig. 2). Figure 3 shows the results of microprobe analysis of carbon distribution in samples after experiments no. 2153 (duration 24 h) and no. 2161 (duration 16 h).
These results indicate an active interaction of carbon with Fe on the surface of the samples with the formation of Fe3C carbide in the ampoules and a diffusion distribution of C in the depth of the samples (Fig. 3). Note the characteristic features of the results associated with different duration of experiments and different ampoule material. In a 24-h experiment (no. 2153) (Fe in a molybdenum ampoule), the reaction of Fe3C formation is realized to a depth of Fe sample by about 50–70 μm (Fig. 3a). Whereas in a sapphire ampoule, the reaction of the formation of Fe3C at the parameters of this experiment is realized only on the surface of the Fe sample (Fig. 3c). In the 16-h experiment (no. 2161) (Fe in a molybdenum and sapphire ampoule), the reaction of Fe3C formation does not take place (Figs. 3b, 3d). These results testify, first of all, to the kinetic features of the iron carburitization reaction at high methane pressures. And on the other hand, they can testify to the different catalytic abilities of Fe–Mo and Fe–sapphire pairs.
Reactions controlling interaction of Fe with methane:
Subtracting (2) from (1) we obtain an equation describing the reaction in a fluid in the presence of a metallic phase:
Under conditions of carbon saturation (a(C) = 1), the equilibrium constant of reaction (3) is expressed:
In (4) ∆Go(3) is the standard free energy of the reaction (3) at temperature T, R = 8.314 J/(K mol) is the universal gas constant, and f(i) is the fugacity of the corresponding fluid particle:
where f o(i) is the fugacity of pure gas i at the corresponding T and P, X(i) is the mole fraction of the gas in the fluid, γ(i) is the coefficient of activity i of the gas in the binary fluid CH4–H2.
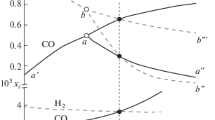
Figure 4 shows the calculated curve of the dependence of the composition of the binary fluid (X(CH4) + X(H2) = 1) on the absolute temperature at a fixed pressure of 100 MPa under conditions of carbon saturation. The volatility of pure gases was calculated by the work of [13], and the values of γ(i) were calculated by the work of ([14], Eq. (8)).
The Fig. 4 shows that as the temperature rises, there should be an increasingly less methane-rich fluid in the presence of free carbon. Figure 4 also shows the result of determining the composition of the fluid phase from experiment no. 2153. It can be seen that the calculation is in good agreement with the experimental definition.
Our results demonstrate that increasing pressure decreases significantly hydrogen yield during methane conversion on metallic iron. Carbon saturation of Fe is not completed in the 24 hours runs. Using molibdenum capsules facilitates methane decomposition.
Change history
21 April 2024
An Erratum to this paper has been published: https://doi.org/10.1134/S1028334X23070292
REFERENCES
B. J. Wood, Science 278, 1727 (1997).
A. A. Marakushev and S. A. Marakushev, Space Time 1, 98–118 (2010).
R. Sweeney, Solid State Ionics 97, 393–397 (1997).
Q. Williams and R. J. Hemley, Annu. Rev. Earth Planet. Sci. 29, 365–418 (2001).
O. Narygina, L. S. Dubrovinsky, C. A. McCammon, et al., Earth Planet. Sci. Lett. 307, 409–414 (2011).
G. Morard, D. Andrault, D. Antonangeli, et al., Earth Planet. Sci. Lett. 473, 94–103 (2017).
K. D. Litasov, A. F. Shatskiy, and E. Ohtani, Geochem. Int. 54, 914–921 (2016).
L. Y. Aranovich, E. S. Persikov, P. G. Bukhtiyarov, et al., Petrology 29 (6), 696–702 (2021).
H. Wang, G. Li, J. Ma, et al., RSC Adv. 7, 3921–3927 (2017).
N. V. Galaktionova, Hydrogen in Metals (Metallurgiya, Moscow, 1967) [in Russian].
H. Sugimoto and Y. Fukai, Acta Metall. Mater. 40 (9), 2327–2336 (1992).
B. V. Oleynikov, A. V. Okrugin, M. D. Tomshin, et al., Native Metals Formation in Mafic Rocks of the Siberian Platform (Yakutsk Office, Siberian Branch, USSR Acad. Sci., Yakutsk, 1985) [in Russian].
S. V. Churakov and M. Gottschalk, Geochim. Cosmochim. Acta 67, 2397–2414 (2003).
L. Ya. Aranovich, Petrology 21 (6), 539–550 (2013).
ACKNOWLEDGMENTS
The authors express their acknowledgments to S.G. Naimushin (GEOKHI RAS) for chromatographic analyses of the fluid phase and dissolved fluid components in samples after experiments.
Funding
The study was funded by the Russian Science Foundation, project 22-27-00124.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Due to a retrospective Open Access order.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aranovich, L.Y., Persikov, E.S., Bukhtiyarov, P.G. et al. Some Features of the Process of Interaction of Iron with Methane at a Temperature of 900°C and a Pressure of 100 MPa. Dokl. Earth Sc. 512, 819–823 (2023). https://doi.org/10.1134/S1028334X2360113X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1028334X2360113X