Abstract
The metal electrodeposition into the nanopores of template of porous anodic alumina type under the conditions of mixed kinetics of metal deposition is studied theoretically using analytical and numerical methods. Two main periods of the process are studied: the non-steady-state formation of diffusion layer in the template pores and much longer process of pore filling with the metal. The effect of nonlinearity of the concentration dependence of the exchange current density of metal electrodeposition on the variation of the current density with the time during the diffusion layer formation and pore filling with the metal is studied.






Similar content being viewed by others
REFERENCES
Whitney, T.M., Jiang, J.S., Searson, P.C., and Chien, C.L., Fabrication and magnetic properties of arrays of metallic nanowires, Science, 1993, vol. 261, p. 1316.
Banerjee, S., Dan, A., and Chakravorty, D., Review synthesis of conducting nanowires, J. Mater. Sci., 2002, vol. 37, p. 4261.
Li, Y., Qian, F., Xiang, J., and Lieber, C.M., Nanowire electronic and optoelectronic devices, Mater. Today, 2006, vol. 9, p. 18.
Davydov, A.D. and Volgin, V.M., Template electrodeposition of metals. Review, Russ. J. Electrochem., 2016, vol. 52, p. 806.
Possin, G.E., A method for forming very small diameter wires, Rev. Sci. Instrum., 1970, vol. 41, p. 772.
Lee, W. and Park, S.-J., Porous anodic aluminum oxide: Anodization and templated synthesis of functional nanostructures, Chem. Rev., 2014, vol. 114, p. 7487.
Proenca, M.P., Sousa, C.T., Ventura, J., Vazquez, M., and Araujo, J.P., Ni growth inside ordered arrays of alumina nanopores: enhancing the deposition rate, Electrochim. Acta, 2012, vol. 72, p. 215.
Napolskii, K.S., Roslyakov, I.V., Eliseev, A.A., Petukhov, D.I., Lukashin, A.V., Chen, S.-F., Liu, C.-P., and Tsirlina, G.A., Tuning the microstructure and functional properties of metal nanowire arrays via deposition potential, Electrochim. Acta, 2011, vol. 56, p. 2378.
Schwanbeck, H. and Schmidt, U., Preparation and characterization of magnetic nanostructures using filtration membranes, Electrochim. Acta, 2000, vol. 45, p. 4389.
Fedorov, F.S., Dunne, P., Gebert, A., and Uhlemann, M., Influence of Cu2+ ion concentration on the uniform electrochemical growth of copper nanowires in ordered alumina template, J. Electrochem. Soc., 2015, vol. 162, p. D568.
Shin, S., Kong, B.H., Kim, B.S., Kim, K.M., Cho, H.K., and Cho, H.H., Over 95% of large-scale length uniformity in template-assisted electrodeposited nanowires by subzero-temperature electrodeposition, Nanoscale Res. Lett., 2011, vol. 6, p. 467.
Valizadeh, S., George, J.M., Leisner, P., and Hultman, L., Electrochemical deposition of Co nanowire arrays: quantitative consideration of concentration profiles, Electrochim. Acta, 2001, vol. 47, p. 865.
Schuchert, I.U., Toimil Molares, M.E., Dobrev, D., Vetter, J., Neumann, R., and Martin, M., Electrochemical copper deposition in etched ion track membranes. Experimental results and a qualitative kinetic model, J. Electrochem. Soc., 2003, vol. 150, p. C189.
Philippe, L., Kacem, N., and Michler, J., Electrochemical deposition of metals inside high aspect ratio nanoelectrode array: analytical current expression and multidimensional kinetic model for cobalt nanostructure synthesis, J. Phys. Chem. C, 2007, vol. 111, p. 5229.
Lopes, M.C., de Oliveira, C.P., and Pereira, E.C., Computational modeling of the template-assisted deposition of nanowires, Electrochim. Acta, 2008, vol. 53, p. 4359.
Bograchev, D.A., Volgin, V.M., and Davydov, A.D., Simple model of mass transfer in template synthesis of metal ordered nanowire arrays, Electrochim. Acta, 2013, vol. 96, p. 1.
Bograchev, D.A., Volgin, V.M., and Davydov, A.D., Simulation of inhomogeneous pores filling in template electrodeposition of ordered metal nanowire arrays, Electrochim. Acta, 2013, vol. 112, p. 279.
Bograchev, D.A., Volgin, V.M., and Davydov, A.D., Modeling of metal electrodeposition in the pores of anodic aluminum oxide, Russ. J. Electrochem., 2015, vol. 51, p. 799.
Bograchev, D.A. and Davydov, A.D., Effect of applied temperature gradient on instability of template-assisted metal electrodeposition, Electrochim. Acta, 2019, vol. 296, p. 1049.
Shin, S., Al-Housseiny, T.T., Kim, B.S., Cho, H.H., and Stone, H.A., The race of nanowires: morphological instabilities and a control strategy, Nano Lett., 2014, vol. 14, p. 4395.
Konishi, Y., Motoyama, M., Matsushima, H., Fukunaka, Y., Ishii, R., and Ito, Y., Electrodeposition of Cu nanowire arrays with a template, J. Electroanal. Chem., 2003, vol. 559, p. 149.
Blanco, S., Vargas, R., Mostany, J., Borrás, C., and Scharifker, B.R., Modeling the growth of nanowire arrays in porous membrane templates, J. Electrochem. Soc., 2014, vol. 161, p. E3341.
Bograchev, D.A. and Davydov, A.D., The role of common outer diffusion layer in the metal electrodeposition into template nanopores, Electrochim. Acta, 2021, vol. 367, p. 137405.
Bograchev, D.A., Volgin, V.M., and Davydov, A.D., Mass transfer during metal electrodeposition into the pores of anodic aluminum oxide from a binary electrolyte under the potentiostatic and galvanostatic conditions, Electrochim. Acta, 2016, vol. 207, p. 247.
Bograchev, D.A. and Davydov, A.D., The shape of end-face surface of a wire growing in a template nanopore, J. Electroanal. Chem., 2021, vol. 900, p. 115709.
Newman, J. and Thomas-Alyea, K.E., Electrochemical Systems, 2004.
Shampine, L.F., Solving 0 = F(t,y(t),y′(t)) in matlab, J. Numer. Math., 2002, vol. 10, p. 291.
Gileadi, E., Kirowa-Eisner, E., and Penciner, J., Interfacial Electrochemistry: An Experimental Approach, New York: Addison-Wesley, Advanced Book Program, 1975.
Skeel, R.D. and Berzins, M., A method for the spatial discretization of parabolic equations in one space variable, SIAM J. Sci. Stat. Comput., 1990, vol. 11, p. 1.
Bograchev, D., Influence of diffusion through a porous film under electrode surface in chronoamperometry problems, Defect Diffus. Forum, Trans. Tech. Publ., 2021, vol. 413, p. 84.
Funding
The work was performed with support of the Ministry of Science and Higher Education of Russian Federation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by T. Kabanova
APPENDIX
APPENDIX
Derivation of Equation for Metal Electrodeposition into Template Nanopores in the Quasi-Steady-State Approximation
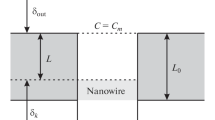
Parameter c0M/ρ controls the ratio between the characteristic diffusion time and the deposition time [24]. The ratio is small for the majority of aqueous solutions. Therefore, it can be always assumed that the deposition proceeds in the steady-state diffusion mode, except for a relatively short initial period of time of reaching the steady-state concentration profile.
Following the works [16, 23], we derive the equation in the quasi-steady-state approximation. Let us write the Fick’s laws for the outer diffusion layer and the diffusion layer inside a pore:
where cs is the concentration at the template surface and cp is the concentration at the pore bottom. The porosity ε in equation (A2) is introduced to match the current density inside the pores and in the outer diffusion layer.
Equations (A1) and (A2) enable us to express the concentration at the pore bottom cp excluding the concentration at the template surface cs. To do this, equation (A1) is multiplied by δ/(Dz+F), equation (A2) is multiplied by L/ε (Dz+F), and thus obtained equations are combined. As a result, we obtain:
Assume that the kinetics of electrodeposition is described by the Tafel equation. Then, taking into account the concentration at the pore bottom and porosity ε, the equation for the current density of metal deposition can be written as follows:
Introducing the kinetic length δk = \(\frac{{DF{{z}_{ + }}{{c}_{0}}}}{{{{i}_{0}}}}\exp \left( {\frac{{{{a}_{{\text{c}}}}F{{\eta }}}}{{RT}}} \right),\) equation (A3) can be written in the form similar to (A1) and (A2):
Concentrations cp and cs can be excluded from (A1) using (A2) and (A3):
Rights and permissions
About this article
Cite this article
Bograchev, D.A., Kabanova, T.B. & Davydov, A.D. Analysis of Effect of Concentration Dependence of Exchange Current on Metal Electrodeposition into Template Nanopores. Russ J Electrochem 59, 651–659 (2023). https://doi.org/10.1134/S1023193523090045
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193523090045