Abstract
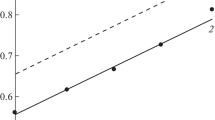
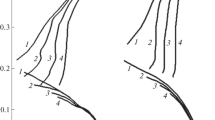
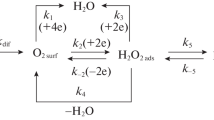
Theoretical aspects of low-carbon steel corrosion in H3PO4 solutions containing FePO4 are considered. In the system under study, reactions of iron with the acid solution and Fe(III) salt are thermodynamically allowed. The oxidizing power of this medium, characterized by the Fe(III)/Fe(II) couple redox potential, is mainly determined by its anionic composition. Phosphate anions of a corrosive medium bind Fe(III) cations into complex compounds, reducing their oxidizing ability. In H3PO4 solutions containing FePO4 and Fe3(PO4)2, the dependence of the system’s redox potential on the Fe(III) and Fe(II) cation relative content is poorly described by the Nernst equation, which is due to the nonequivalent complex formation of these cations with phosphate anions. Analysis of the effect of the studied media convection on the low-carbon steel electrode reactions allowed revealing some of their features. In a FePO4-containing H3PO4 solution, kinetically controlled partial reactions of iron anodic ionization and H+ cathodic reduction, as well as diffusion-controlled Fe(III) cation cathodic reduction, occur on the steel. The FePO4 accelerating effect on the steel corrosion in H3PO4 solution is due only to the Fe(III) reduction but does not affect the H+ reduction and the iron ionization. The value of the Fe(III)-cation diffusion coefficient in the studied corrosive medium was experimentally determined from the data of cyclic voltammetry of the Pt electrode therein and the results of the studying of the cathodic reaction of a steel disk electrode at different rotation velocities. The data on the low-carbon steel corrosion in the flow of the studied media, obtained from the metal samples mass loss, are in full agreement with the results of the study of the electrode partial reactions. An accelerating effect of FePO4 on the steel corrosion in H3PO4 solutions is observed. In this environment, steel corrosion is determined by the convective factor, which is typical of processes with diffusion control. The empirical dependence of the steel corrosion rate on the medium flow intensity is described by the linear dependence k = kst + λw1/2, where kst is the steel corrosion rate in a static medium, w is the rotation velocity of the propeller stirrer that creates the medium flow, λ is the empirical coefficient.






Similar content being viewed by others
REFERENCES
Kuzin, A.V., Gorichev, I.G., Shelontsev, V.A., Kuzmenko, A.N., Plakhotnaia, O.N., and Ovsyannikova, L.V., The Role of a Complex Formation in the Dissolution of Iron Oxides in Orthophosphoric Acid, Moscow Univ. Chem. Bull., 2021, vol. 76, no. 6, p. 398. https://doi.org/10.3103/S0027131421060055
Prodan, I.E., Yeshchenko, L.S., and Pechkovsky, V.V., Study of the crystallization of iron phosphates in the system iron(III)—phosphoric acid—water, Russ. J. Inorg. Chem. (in Russian), 1989, vol. 34, no. 7, p. 1860.
Barthel, J. and Deiss, R., The limits of the Pourbaix diagram in the interpretation of the kinetics of corrosion and cathodic protection of underground pipelines, Mater. and Corros., 2021, vol. 72, no. 3, p. 434. https://doi.org/10.1002/maco.202011977
Huang, H.-H., The E h–pH Diagram and Its Advances, Metals, 2016, vol. 6, no. 1, p. 23. https://doi.org/10.3390/met6010023
Perry, S.C., Gateman, S.M., Stephens, L.I., Lacasse, R., Schulz, R., and Mauzeroll, J., Pourbaix Diagrams as a Simple Route to First Principles Corrosion Simulation, J. Electrochem. Soc., 2019, vol. 166, no. 11, p. C3186. https://doi.org/10.1149/2.0111911jes
Pourbaix, M., Atlas of Electrochemical Equilibria in Aqueous Solutions, 2nd English Edition, Houston: National Association of Corrosion Engineers, 1974, p. 307.
Wermink, W.N. and Versteeg, G.F., The Oxidation of Fe(II) in Acidic Sulfate Solutions with Air at Elevated Pressures. Part 1. Kinetics above 1 M H2SO4, Ind. Eng. Chem. Res., 2017, vol. 56, no. 14, p. 3775. https://doi.org/10.1021/acs.iecr.6b04606
Wermink, W.N. and Versteeg, G.F., The Oxidation of Fe(II) in Acidic Sulfate Solutions with Air at Elevated Pressures. Part 2. Influence of H2SO4 and Fe(III), Ind. Eng. Chem. Res., 2017, vol. 56, no. 14, p. 3789. https://doi.org/10.1021/acs.iecr.6b04641
Zakharov, V.A., Songina, O.A., and Bekturova, G.B., Real potentials of oxidation–reduction systems (Overview), Zh. Anal. Khim. (in Russian), 1976, vol. 31, no. 11, p. 2212.
Kaesche, H., Die Korrosion der Metalle. Physikalischchemische Prinzipien und Aktuelle Probleme, Berlin: Springer, 1979.
Antropov, L.I. Theoretical Electrochemistry (in Russian), Moscow, Vysshaya Shkola, 1965, p. 348–380.
Bockris, J.O'M., Drazic, D., and Despic, A.R., The electrode kinetics of the deposition and dissolution of iron, Electrochim. Acta, 1961, vol. 4, no. 2–4, p. 325. https://doi.org/10.1016/0013-4686(61)80026-1
Katrevich, A.N., Florianovich, G.M., and Kolotyrkin, Ya.M., Elucidation of the kinetic parameters of the reaction of active dissolution of iron in phosphate solutions, Prot. Met. (in Russian), 1974, vol. 10, no. 4, p. 369.
Reshetnikov, S.M. and Makarova, L.L., Kinetics and mechanism of cathodic and anodic processes that determine acid corrosion of metals in the area of active dissolution, In: Redox and Adsorption Processes on the Solid Metal Surfaces (in Russian), Udmurt State Univ., Izhevsk, 1979, p. 25–49.
Avdeev, Ya.G. and Andreeva, T.E. Characteristics of the Mechanism of Corrosion of Low-Carbon Steels in Acid Solutions Containing Fe(III) Salts, Russ. J. Phys. Chem. A., 2021, vol. 95, no. 6, p. 1128. https://doi.org/10.1134/S0036024421060029
Avdeev, Ya.G. and Andreeva, T.E., Mechanism of Steel Corrosion in Inhibited Acid Solutions Containing Iron(III) Salts, Russ. J. Phys. Chem. A., 2022, vol. 96, no. 2, p. 423. https://doi.org/10.1134/S0036024422020030
Avdeev, Ya.G., Andreeva, T.E., Panova, A.V., and Kuznetsov, Yu.I., Effect of anionic composition of solutions of mineral acids containing Fe(III) on their oxidizing properties, Int. J. Corros. Scale Inhib., 2019, vol. 8, no. 1, p. 139. https://doi.org/10.17675/2305-6894-2019-8-1-12
Lurie, Yu.Yu., Spravochnik po analiticheskoy khimii (Handbook of Analytical Chemistry) (in Russian), Moscow, Khimiya, 1971, p. 255–265.
Kim, M.S., Kim, C.H., and Sohn, Y.S., Complex Formation Between Ferric Ion and Phosphoric Acid, J. Korean Chem. Soc., 1975, vol. 19, no. 5, p. 325.
Filatova, L.N., Vendilo, A.G., and Sandu, R.A., Chemical Forms of Iron(III) in Solutions of Orthophosphoric Acid as Probed by Electronic Absorption Spectroscopy, Russ. J. Inorg. Chem., 2012, vol. 57, no. 9, p. 1272. https://doi.org/10.1134/S0036023612090057
Plambeck, J.A. Electroanalytical Chemistry: Basic Principles and Applications, New York : Wiley, 1982.
Belqat, B., Laghzizil, A., Elkacimi, K., Bouhaouss, A., and Belcadi, S., Fluoride effect on the electrochemical behaviour of the Fe(III)/Fe(II) system in H3PO4 + H2O + HF, J. Fluorine Chem., 2000, vol. 105, p. 1. https://doi.org/10.1016/S0022-1139(00)00256-6
Pleskov, Yu.V. and Filinovskii, V.Yu., The Rotating Disk Electrode, New York: Consultants Bureau, 1976.
Du, C., Tan, Q., Yin, G., and Zhang, J., Rotating Disk Electrode Method, In Rotating Electrode Methods and Oxygen Reduction Electrocatalysts, Eds. Xing, W., Yin, G., and Zhang, J., Elsevier B.V., 2014, p. 171–198. https://doi.org/10.1016/B978-0-444-63278-4.00005-7
Jia, Z., Yin, G., and Zhang, J., Rotating Ring-Disk Electrode Method, In Rotating Electrode Methods and Oxygen Reduction Electrocatalysts, Eds. Xing, W., Yin, G., and Zhang, J., Elsevier B.V., 2014, p. 199–229. https://doi.org/10.1016/B978-0-444-63278-4.00006-9
Xing, W., Yin, M., Lv, Q., Hu, Y., Liu, C., and Zhang, J., Oxygen solubility, diffusion coefficient, and solution viscosity, In Rotating Electrode Methods and Oxygen Reduction Electrocatalysts, Eds. Xing, W., Yin, G., and Zhang, J., Elsevier B.V., 2014, p. 1–31. https://doi.org/10.1016/B978-0-444-63278-4.00001-X
Funding
This work is carried out as R&D “Chemical material resistance, protection of metals and other materials against corrosion and oxidation” (2022–2024), the Integrated National Information System reg. no. 122011300078-1, the inventory no. FFZS-2022-0013.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by Yu. Pleskov
Rights and permissions
About this article
Cite this article
Avdeev, Y.G., Panova, A.V. & Andreeva, T.E. Corrosion of Low-Carbon Steel in a Flow of Phosphoric Acid Solution Containing Iron(III) Phosphate. Russ J Electrochem 59, 512–523 (2023). https://doi.org/10.1134/S1023193523070030
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193523070030