Abstract
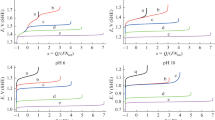
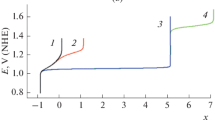
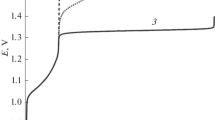
Variations of the indicator electrode potential under zero current condition, E, and of the (quasi)equilibrium composition of the aqueous solution (which initially contained 0.5 M chloride anion) in the anode chamber of a model electrolytic cell were calculated, provided that the pH of the solution is kept constant (pH 0) and that the same total number of Cl atoms in all chlorine compounds (including the gas phase above it) is maintained. Theoretical analysis is carried out for five different hypotheses regarding the possible depth of electrolysis and the nature of the occurring processes. The hypotheses are: (a) no formation of chlorine compounds with oxidation states above +1, i.e., the electrolysis produced molecular chlorine Cl2 as a solute and in the gas space above the solution \({\text{Cl}}_{{\text{2}}}^{{{\text{gas}}}}{\text{,}}\) trichloride anion \({\text{Cl}}_{3}^{ - },\) as well as dissolved HClO and ClO– (in addition to the retaining amount of Cl–); (b) in addition to the compounds indicated for case (a), chlorine compounds are formed in solution in the +3 oxidation state, i.e. HClO2 and \({\text{ClO}}_{2}^{ - }{\text{;}}\) (c) in addition to the compounds listed for case (b), chlorine compounds are formed in the +4 oxidation state, i.e. dissolved ClO2 and \({\text{ClO}}_{{\text{2}}}^{{{\text{gas}}}}\) in the gas phase; (d) the process proceeds with formation of both the chlorate ion \(\left( {{\text{ClO}}_{3}^{ - }} \right)\) and the chlorine compounds of lower oxidation states in solution and in gas phase indicated above for case (c) (Cl–, \({\text{Cl}}_{3}^{ - },\) Cl2, \({\text{Cl}}_{{\text{2}}}^{{{\text{gas}}}}{\text{,}}\) HClO, ClO–, HClO2, \({\text{ClO}}_{2}^{ - }{\text{,}}\) ClO2, and \({\text{ClO}}_{{\text{2}}}^{{{\text{gas}}}}\)); (e) in addition to the components indicated for variant (d), perchlorate anion \(\left( {{\text{ClO}}_{4}^{ - }} \right)\) can also be formed. All electrochemical and chemical reactions involving the chlorine-containing species which are taken into account within the framework of each of the options (a), (b), (c), (d) or (e) are assumed to be in (quasi)equilibrium state. Predictions for all these five hypotheses on the relationship between the redox-charge of the system, Q (or the average oxidation state of chlorine atoms, x), and the indicator electrode potential, E, as well as on the dependence of the system’s composition (concentrations of all compounds) on either x or E are derived. Approaches for the experimental determining of the variant for the evolution of the Cl-containing anolyte composition in the course of electrolysis are proposed.



Similar content being viewed by others
REFERENCES
Chen, R., Trieu, V., Schley, B., Natter, H., J. Kintrup, J., Bulan, A., Weber, R., and Hempelmann, R., Anodic Electrocatalytic Coatings for Electrolytic Chlorine Production: A Review, Z. Phys. Chem., 2013, Bd. 227, S. 651. https://doi.org/10.1524/zpch.2013.0338
Crook, J. and Mousavi, A., The chlor-alkali process: A review of history and pollution, Environmental Forensics, 2016, vol. 17, no. 3, p. 21. https://doi.org/10.1080/15275922.2016.1177755
Kelsall, G.H., Welham, N.J., and Diaz, M.A., Thermodynamics of Cl–H2O, Br–H2O, I–H2O, Au–Cl–H2O, Au–Br–H2O and Au–I–H2O systems at 298 K, J. Electroanal. Chem., 1993, vol. 361, no. 1–2, p. 13. https://doi.org/10.1016/0022-0728(93)87034-S
Pourbaix, M., Atlas of Electrochemical Equilibria in Aqueous Solutions, Houston: National Association of Corrosion Engineers, 1974. Section 20.2 (Chlorine), p. 590–603.
Vogt, H., Balej, J., Bennett, J.E., Wintzer, P., Sheikh, S.A., Gallone, P., Vasudevan, S., and Pelin, K., Chlorine Oxides and Chlorine Oxygen Acids, in Ullmann’s Encyclopedia of Industrial Chemistry, Ullmann, F., Ed., Berlin: Wiley Online Library, 2010, p. 622. https://doi.org/10.1002/14356007.a06_483.pub2
Viswanathan, K. and Tilak, B.V., Chemical, electrochemical, and technological aspects of sodium chlorate manufacture, J. Electrochem. Soc., 1984, vol. 131, no. 7, p. 1551. https://doi.org/10.1149/1.2115908
Wulff, J. and Cornell, A., Cathodic current efficiency in the chlorate process, J. Appl. Electrochem., 2007, vol. 37, p. 181. https://doi.org/10.1007/s10800-006-9263-3
Vacca, A., Mascia, M., Palmas, S., Mais, L., and Rizzardini, S., On the formation of bromate and chlorate ions during electrolysis with boron doped diamond anode for seawater treatment, J. Chem. Technol. Biotechnol., 2013, vol. 88, no. 12, p. 2244. https://doi.org/10.1002/jctb.4095
Karlsson, R.K.B. and Cornell, A., Selectivity between Oxygen and Chlorine Evolution in the Chlor-Alkali and Chlorate Processes, Chem. Rev., 2016, vol. 116, no. 5, p. 2982. https://doi.org/10.1021/acs.chemrev.5b00389
Wang, T.X. and Margerum, D.W., Kinetics of Reversible Chlorine Hydrolysis: Temperature Dependence and General-Acid/Base-Assisted Mechanisms, Inorg. Chem., 1994, vol. 33, no. 6, p. 1050. https://doi.org/10.1021/ic00084a014
Alves, W.A., Téllez, C.A., Sala, S.O., Santos, P.S., and Faria, R.B., Dissociation and rate of proton transfer of HXO3 (X = Cl, Br) in aqueous solution determined by Raman spectroscopy, J. Raman Spectroscopy, 2001, vol. 32, p. 1032. https://doi.org/10.1002/jrs.794
Tarasevich, M.R., Sadkowsky, A., and Yeager, E., Oxygen Electrochemistry, In Comprehensive Treatise of Electrochemistry, Horsman, P., Conway, B.E., and Yeager, E., Eds., Boston: Springer, 1983, p. 301.
Reier, T., Oezaslan, M., and Strasser, P., Electrocatalytic Oxygen Evolution Reaction (OER) on Ru, Ir, and Pt Catalysts: A Comparative Study of Nanoparticles and Bulk Materials, ACS Catal., 2012, vol. 2, p. 1765. https://doi.org/10.1021/cs3003098
Mussini, T. and Longhi, P., The Halogens. Bromine, in Standard potentials in aqueous solutions, Bard, A.J., Parsons, R. and Jordan J., Eds., New York: Marcel Dekker, 1985, p. 78. https://books.google.ru/books?id= fuJV1H18KtEC&pg=PA67&hl=ru&source=gbs_toc_r& cad=4#v=onepage&q&f=false
Lide, D.R., CRC handbook of Chemistry and Physics, vol. 84, New York: CRC press, 2004.
Skrabal, A. and Schreiner, H., Die Reduktionsgeschwindigkeit der Chlorsäure und Bromsäure, Monatshefte für Chemie und verwandte Teile anderer Wissenschaften, 1934, vol. 65, no. 1, p. 213. https://doi.org/10.1007/bf01522061
Bray, W.C., An oxide of iodine, I2O2, an intermediate compound, J. Amer. Chem. Soc., 1930, vol. 52, p. 3580. https://doi.org/10.1021/ja01372a017
Taube, H. and Dodgen, H., Applications of Radioactive Chlorine to the Study of the Mechanisms of Reactions Involving Changes in the Oxidation State of Chlorine, J. Amer. Chem. Soc., 1949, vol. 71, no. 10, p. 3330. https://doi.org/10.1021/ja01178a016
Lenzi, F. and Rapson, W.H., Effets ioniques spécifiques sur le taux de formation du ClO2 par la réaction chlorure–chlorate, Canad. J. Chem., 1968, vol. 46, no. 6, p. 979. https://doi.org/10.1139/v68-160
Schmitz, G., Kinetics and mechanism of the iodate–iodide reaction and other related reactions, Phys. Chem. Chem. Phys., 1999, vol. 1, no. 8, p. 1909. https://doi.org/10.1039/a809291e
Sant’Anna, R.T.P., Santos, C.M.P., Silva, G.P., Ferreira, R.J.R., Oliveira, A.P., Côrtes, C.E.S., and Faria, R.B., Kinetics and mechanism of chlorate-chloride reaction, J. Brazilian Chem. Soc., 2012, vol. 23, no. 8, p. 1543. https://doi.org/10.1590/S0103-50532012005000017
Pillai, K.C., Kwon, T.O., Park, B.B., and Moon, I.S., Studies on process parameters for chlorine dioxide production using IrO2 anode in an un-divided electrochemical cell, J. Hazardous Mater., 2009, vol. 164, nos. 2–3, p. 812. https://doi.org/10.1016/j.jhazmat.2008.08.090
Pillai, K.C., Kwon, T.O., Park, B.B., and Moon, I.S., Using RuO2 anode for chlorine dioxide production in an un-divided electrochemical cell, Water Sci. Tchnol., 2010, vol. 61, no. 8, p. 2151. https://doi.org/10.2166/wst.2010.131
Tian, M., Li, Y.Y., Sun, H.C., Yang, L.J., and Li, Z.L., Preparation of Chlorine Dioxide by Electrocatalytic Reduction of Sodium Chlorate, Advanced Mater. Res., 2013, vols. 781–784, p. 342. DOI 10.4028/www.scientific.net/amr.781-784.342
Raspi, G. and Pergola, F., Voltammetric behaviour of chlorites and chlorine dioxide on a platinized-platinum microelectrode with periodical renewal of the diffusion layer and its analytical applications, J. Electroanal. Chem., 1969, vol. 20, no. 3, p. 419. https://doi.org/10.1016/s0022-0728(69)80171-3
Pergola, F., Guidelli, R., and Raspi, G., Potentiostatic study of heterogeneous chemical reactions. ClO2–ClO2–Cl-system on platinized platinum, J. Amer. Chem. Soc., 1970, vol. 92, no. 9, p. 2645. https://doi.org/10.1021/ja00712a010
Lipsztajn, M., Electrolytic protection of chlorine dioxide, Patent 4,767,510 (USA), 1988.
Sinkaset, N., Nishimura, A.M., Pihl, J.A., and Trogler, W.C., Slow Heterogeneous Charge Transfer Kinetics for the ClO2-/ClO2 Redox Couple at Platinum, Gold, and Carbon Electrodes. Evidence for Nonadiabatic Electron Transfer, J. Phys. Chem. A, 1999, vol. 103, no. 49, p. 10461. https://doi.org/10.1021/jp992693f
Gomez-Gonzalez, A., Ibanez, J.G., Vasquez-Medrano, R., Zavala-Araiza, D., and Paramo-Garcia, U., Electrochemical Paired Convergent Production of ClO, ECS Trans., 2009, vol. 20, no. 1, p. 91. https://doi.org/10.1149/1.3268376
Gomez-Gonzalez, A., Ibanez, J.G., Vasquez-Medrano, R.C., Paramo-Garcia, U., and Zavala-Araiza, D., Cathodic Production of ClO2 from NaClO3, J. Electrochem. Soc., 2009, vol. 156, no. 7, p. E113. https://doi.org/10.1149/1.3121588
Schmitz, G. and Rooze, H., Mécanisme des réactions du chlorite et du dioxyde de chlore. 5. Cinétique de la réaction chlorite–bromure, Canad. J. Chem., 1987, vol. 65, no. 3, p. 497. https://doi.org/10.1139/v87-086
Ni, Y. and Yin, G., Disproportionation of Chlorous Acid at a Strong Acidity, Industrial & Engineering Chem. Res., 1998, vol. 37, no. 6, p. 2367. https://doi.org/10.1021/ie970608p
Tolmachev, Y.V., Pyatkivskiy, A., Ryzhov, V.V., Konev, D.V., and Vorotyntsev, M.A., Energy cycle based on a high specific energy aqueous flow battery and its potential use for fully electric vehicles and for direct solar-to-chemical energy conversion, J. Solid State Electrochem., 2015, vol. 19, p. 2711. https://doi.org/10.1007/s10008-015-2805-z
Vorotyntsev, M.A., Antipov, A.E., and Konev, D.V., Bromate anion reduction: novel autocatalytic (EC") mechanism of electrochemical processes. Its implication for redox flow batteries of high energy and power densities, Pure Appl. Chem., 2017, vol. 89, no. 10, p. 1429. https://doi.org/10.1515/pac-2017-0306
Petrov, M.M., Loktionov, P.A., Konev, D.V., Antipov, A.E., Astafiev, E.A., and Vorotyntsev, M.A., Evolution of Anolyte Composition in the Oxidative Electrolysis of Sodium Bromide in a Sulfuric Acid Medium, Russ. J. Electrochem., 2018, vol. 54, p. 1223.
Petrov, M.M., Konev, D.V., Kuznetsov, V.V., Antipov, A.E., Glazkov, A.T., and Vorotyntsev, M.A., Electrochemically driven evolution of Br-containing aqueous solution composition, J. Electroanal. Chem., 2019, vol. 836, p. 125. https://doi.org/10.1016/j.jelechem.2019.01.070
Petrov, M.M., Konev, D.V., Antipov, A.E., Kartashova, N.V., Kuznetsov, V.V., and Vorotyntsev, M.A., Theoretical Analysis of Changes in the Solution Composition during Anodic Electrolysis of Bromide, Russ. J. Electrochem., 2019, vol. 55, p. 1058.
Petrov, M.M., Konev, D.V., Antipov, A.E., Kartashova, N.V., Kuznetsov, V.V., and Vorotyntsev, M.A., Theoretical Analysis of Changes in the System’s Composition in the Course of Oxidative Electrolysis of Bromide Solution: pH Dependence, Russ. J. Electrochem., 2020, vol. 56, p. 883.
Vorotyntsev, M.A., Konev, D.V., and Tolmachev, Y.V., Electroreduction of halogen oxoanions via autocatalytic redox mediation by halide anions: novel EC" mechanism. Theory for stationary 1D regime, Electrochim. Acta, 2015, vol. 173, p. 779. https://doi.org/10.1016/j.electacta.2015.05.099
Modestov, A.D., Konev, D.V., Antipov, A.E., Petrov, M.M., Pichugov, R.D., and Vorotyntsev, M.A., Bromate electroreduction from sulfuric acid solution at rotating disk electrode: experimental study, Electrochim. Acta, 2018, vol. 259, p. 655. https://doi.org/10.1016/j.electacta.2017.10.199
Modestov, A.D., Konev, D.V., Tripachev, O.V., Antipov, A.E., Tolmachev, Y.V., and Vorotyntsev, M.A., A Hydrogen–Bromate Flow Battery for Air-Deficient Environments, Energy Technology, 2018, vol. 6, p. 242. https://doi.org/10.1002/ente.201700447
Modestov, A.D., Konev, D.V., Antipov, A.E., and Vorotyntsev, M.A., Hydrogen-bromate flow battery: can one reach both high bromate utilization and specific power?, J. Solid State Electrochem., 2019, vol. 23, p. 3075. https://doi.org/10.1007/s10008-019-04371-w
Modestov, A.D., Andreev, V.N., Antipov, A.E., and Petrov, M.M., Novel Aqueous Zinc–Halogenate Flow Batteries as an Offspring of Zinc–Air Fuel Cells for Use in Oxygen-Deficient Environment, Energy Technol., 2021, vol. 9, no. 9, p. 1. https://doi.org/10.1002/ente.202100233
Bergman, M.E.H., Iourtchouk, T., and Rollin, J., The occurrence of bromate and perbromate on BDD anodes during electrolysis of aqueous systems containing bromide: first systematic experimental studies, J. Appl. Electrochem., 2011, vol. 41, no. 9, p. 1109. https://doi.org/10.1007/s10800-011-0329-5
Sáez, C., Sánchez-Carretero, A., Cañizares, P., and Rodrigo, M.A., Electrochemical synthesis of perbromate using conductive-diamond anodes, J. Appl. Electrochem., 2010, vol. 40, no. 10, p. 1715. https://doi.org/10.1007/s10800-010-0108-8
Sander, R., Compilation of Henry’s Law Constants for Inorganic and Organic Species of Potential Importance in Environmental Chemistry, Mainz: Max-Planck Inst. Chem., 1999. p. 9–10.
Harris, D.C., Exploring Chemical Analysis, New York: W.H. Freeman, 2009, p. 538.
Fabian, I. and Gordon, G., Complex Formation Reactions of the Chlorite Ion, Inorg. Chem., 1991, vol. 30, p. 3785. https://doi.org/10.1021/ic00019a045
Stanbury, D.M. and Figlar, J.N., Vanishingly slow kinetics of the ClO2/Cl– reaction: its questionable significance in nonlinear chlorite reactions, Coordination Chem. Rev., 1999, vol. 187, no. 1, p. 223. https://doi.org/10.1016/S0010-8545(99)00092-2
Funding
This work was supported by the Russian Science Foundation, project no. 20-63-46041.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by Yu. Pleskov
A tribute to outstanding electrochemist Oleg Aleksandrovich Petrii (1937–2021).
APPENDIX
APPENDIX
The system compositions are calculated basing on thermodynamical relations (А1)–(А11) for equilibria in electrochemical, chemical, and physical transformations of Table 2.
In these relations, we used the following notation: Х for Cl-containing component in aqueous solution (Cl–, Cl2, \({\text{Cl}}_{3}^{ - },\) HClO, ClO–, HClO2, \({\text{ClO}}_{2}^{ - },\) \({\text{ClO}}_{3}^{ - },\) \({\text{ClO}}_{4}^{ - }\)), {X} for its activity, \({\text{Cl}}_{{\text{2}}}^{{{\text{gas}}}}\) and \({\text{ClO}}_{{\text{2}}}^{{{\text{gas}}}}\) for molecular chlorine and its dioxide in gas phase, \(\left\{ {{\text{Cl}}_{{\text{2}}}^{{{\text{gas}}}}} \right\}\) and \(\left\{ {{\text{ClO}}_{{\text{2}}}^{{{\text{gas}}}}} \right\}\) for their activities (in the molecular concentration unities: mol/dm3), Vsol and Vgas for the volumes of solution and the gas phase above it, \(E_{i}^{^\circ }\) for the standard potential of the corresponding electrochemical reaction, Ki for the equilibrium constant of the corresponding chemical transformation or for the transfer to the gas phase (Kgas, с). The dimensionless constants \(K_{3}^{{{\text{gas,c}}}}\) and \(K_{9}^{{{\text{gas,c}}}}\) for the equilibria (3) and (9) between the gas phase and the Cl2 and ClO2 solutes are calculated by using the values of the corresponding Henry constants KH3 and KH9 [M/atm] [46]. We denote the common logarithm as “log”. The parameter A = F/(RT ln 10) = 16.92 V–1 (at a temperature of 298 К).
For the components’ concentrations [Xi] all expressions (А1)–(А11) are valid provided the activity of each component {Xi} has been replaced by [Xi]; the standard potential of each reaction\(E_{i}^{^\circ }\), by the formal potential \({E}_{i}^{{^\circ }'},\); the equilibrium constant for any transformation Ki, by the apparent equilibrium constant \(K_{i}^{'}\) [36]. Further calculations were carried out under the assumption that the components’ activities in all relations in Table 2 have been replaced by the concentrations, while it is thermodynamical quantities of the parameters that were used in these relations.
The Method of Calculation of the System’s Component Concentrations
The calculations were carried out for the known values of the solution рН (рН 0), the chlorine atom summary concentration in the system ctot = 0.5 M, and the volume ratio for the solution and gas phase (in the calculations, we assumed the value Vgas/Vsol = 0.8) for the set of values of the potential Е. This set of the potential values is chosen in such a way that the system’s mean oxidation value therein х varied from –1 to the maximal possible value: up to +7 or up to +5, or up to +4, or up to +3, or up to +1, respectively, in the variants (e), (d), (c), (b), or (а).
For the variant (e), a nonlinear set of 12 algebraic equations (14), (А1)–(А11) is solved for 12 unknown concentrations of the chlorine compounds in Table 1. The calculations for the variants (а), (b), (c), and (d) are carried out by the same scheme, however, with a decreased number of chlorine-containing compounds and upon removing of the same number of their determining conditions of thermodynamical equilibrium. In particular, for the variant (d), in which no perchlorate-anion is formed, its concentration is zeroed, and equation (А11) is excluded from the analysis. For the variant (c), the chlorate-anion \({\text{ClO}}_{3}^{ - }\) and equation (А10) are also excluded; for the variant (b), also ClO2 and \({\text{ClO}}_{{\text{2}}}^{{{\text{gas}}}}\) and equations (А9) and (А8); for the variant (а), also HClO2 and \({\text{ClO}}_{2}^{ - }\), as well as equations (А7) and (А6) for their concentrations.
In all cases, the equations for the concentrations (А1)–(А11) we used in which the рН potential values are assumed to be known, allow expressing all concentrations сi in terms of one of the concentrations, for example, [Cl–]. The substituting of these expressions into equation (14) for the summary number of chlorine atoms brings about to nonlinear algebraic cubic equation for this concentration. The equation was solved numerically for each value of the potential E. Then, using formulas (А1)–(А11) we calculated the concentrations of the rest of components, the system’s redox-charge Q, and the chlorine-atom mean oxidation state х by formulas (15) for the chosen value of the potential Е.
The numerical solution of the nonlinear equation for the chloride-anion concentration [Cl–] (see the preceding paragraph) was carried out as follows. The right-hand part of equation (14) (∑nici) is a sum of concentrations of all system’s components ci (depending on the variant of the calculations, part of concentrations was zeroed, that is, excludes from the summation over i) with positive coefficients ni, where each quantity ci is connected with [Cl–] by a corresponding equation (А1)–(А11) from Table 2. At that, all concentrations ci increased monotonously with the increasing of the concentration [Cl–]. This means that (at any fixed values of рН and Е) the sum of concentrations ∑nici also increased monotonously with the increasing of the concentration [Cl–]. Moreover, the sun is 0 at zero concentration [Cl–] for any values of рН and Е. Wherefrom we derived a conclusion: at any value of the system’s global parameters (рН, Е, and сtot) there exists—moreover, the only possible—solution of equation (14)for [Cl–], that is, the sum of concentrations ∑nici equals сtot. As at any nonzero (positive) value of [Cl–] the rest of the concentrations also is positive, the above-mentioned only possible solution falls within the interval: 0 < [Cl–] < сtot, because at its left-hand boundary the sum ∑nici is 0; at its right-hand boundary, a fortiori it exceeds сtot.
To find the solution of equation (14) in this interval, we used the bisection method, that is, the halving of the interval and the calculating of the sum ∑nici for this mid-value of [Cl–], for example, at [Cl–] = 1/2сtot in the first step. When the obtained value of the sum is larger than сtot, the solution of the equation should be looked for at the left-hand half of the interval; when the sum is less than сtot, the root is located at the right-hand half of the interval. This procedure is further repeated for the required half of the interval, and so on, until the preset accuracy of determination of [Cl–] or deviation of the summary concentration, that is, the difference ∑nici – сtot, has been reached.
For the other set of global parameters (рН, Е, and сtot), for instance, for the other value of the potential Е, the same procedure is to be repeated. Eventually, the system’s composition has been found, that is, all considered concentrations ci, as well as the system’s redox-charge Q and the chlorine-atom mean oxidation state х is found using equations (15) as a function of the potential Е at given values of the rest of parameters (рН and сtot).
At sufficiently high positive potentials, the concentration [Cl–] becomes so small that the above-described calculations became impossible because of limited accuracy of the numerical operations. In this case, we used in the calculation the same approach, yet, we chose as a basic unknown the concentration of the compound with positive oxidation degree, e.g., \(\left[ {{\text{ClO}}_{3}^{ - }} \right]\) for the variants (e) and (d) or [ClО2] for the variant (c). There exists a broad interval of intermediate potentials in which we succeeded in the calculating of both unknown quantities whose comparison allowed verifying the identity of the obtained results.
The correctness of the calculations was controlled by the calculating of the summary concentration of chlorine atoms, as well as by verifying the fulfilment of equations (А1)–(А11).
When performing the calculations (at fixed values of рН and ctot) for a set of various potential values, we succeeded in the predicting of the dependence of х on Е (or, which is the same thing, Е on х, see Fig. 1), as well as the system composition on the electrode potential Е (Fig. 3) or on the mean oxidation degree х (Fig. 2) for each evolution variant (а)–(e).
Rights and permissions
About this article
Cite this article
Zader, P.A., Konev, D.V., Gun, J. et al. Theoretical Analysis of System’s Composition Changes in the Course of Electrolysis of Acidic Chloride Aqueous Solution. Russ J Electrochem 58, 869–884 (2022). https://doi.org/10.1134/S1023193522100123
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193522100123