Abstract
Changes in the indicator electrode potential (at zero current) E and (quasi) equilibrium composition of aqueous solution in the anode chamber of the model electrolyzer, which initially contained 0.5 M concentration of bromide anions, provided that the solution was kept at a constant pH and constant (together with the gas phase above it) total number of Br atoms in all its compounds, are calculated. Theoretical analysis was carried out for four different hypotheses regarding the possible extent of electrolysis and the nature of the processes are theoretically analyzed. They are: (1) no formation of bromine compounds with positive oxidation states occurs, i.e., electrolysis only leads to the formation of molecular bromine in its various forms (the dissolved state of Br2, as well as phases of liquid bromine \({\text{Br}}_{{\text{2}}}^{{{\text{liq}}}}\), and bromine vapor in the gas space above the \({\text{Br}}_{{\text{2}}}^{{{\text{vap}}}}\) solution); (2) oxidation of bromide ions leads to the formation of bromine compounds in its oxidation state up to +1 inclusive; (3) the process proceeds with the formation of both bromate ion (BrO3-) and compounds of bromine with lower oxidation states in solution (\({\text{Br}}_{{\text{3}}}^{ - },\) \({\text{Br}}_{{\text{5}}}^{ - },\) Br2, \({\text{Br}}_{{\text{2}}}^{{{\text{liq}}}},\) \({\text{Br}}_{{\text{2}}}^{{{\text{vap}}}},\) BrO–, HBrO); (4) in addition to the components specified in clause (3), the formation of the perbromate anion \(\left( {{\text{BrO}}_{{\text{4}}}^{ - }} \right)\) is also taken into consideration. All electrochemical and chemical reactions involving bromine-containing species have been taken into consideration in the hypothesis framework of the system’s evolution (1), (2), (3), or (4), are assumed to be in a (quasi)equilibrium state. Predictions for all hypotheses (1), (2), (3), or (4) have been compared at three different pH values of the solution (2, 6 and 10 of Br-containing anolyte composition’s evolution in the course of electrolysis.







Similar content being viewed by others
REFERENCES
Ferro, S. and De Battisti, A., The bromine electrode. Part I: Adsorption phenomena at polycrystalline platinum electrodes, J. Appl. Electrochem., 2004, vol. 34, no. 10, p. 981.
Ferro, S., Orsan, C., and De Battisti, A., The bromine electrode. Part II: Reaction kinetics at polycrystalline Pt, J. Appl. Electrochem., 2005, vol. 35, no. 3, p. 273.
Ferro, S., The bromine electrode Part III: reaction kinetics at highly boron-doped diamond electrodes, J. Appl. Electrochem., 2005, vol. 35, no. 3, p. 279.
Bergman, M.E.H., Iourtchouk, T., and Rollin, J., The occurrence of bromate and perbromate on BDD anodes during electrolysis of aqueous systems containing bromide: first systematic experimental studies, J. Appl. Electrochem., 2011, vol. 41, no. 9, p. 1109.
Osuga, T. and Sugino, K., Electrolytic production of bromates, J. Appl. Electrochem., 1957, vol. 104, no. 7, p. 448.
Vacca, A., Mascia, M., Palmas, S., Mais, L., and Rizzardini, S., On the formation of bromate and chlorate ions during electrolysis with boron doped diamond anode for seawater treatment, J. Chem. Technol. Biotechnol., 2013, vol. 88, no. 12, p. 2244–2251.
Cettou, P., Robertson, P.M., and Ibl, N., On the electrolysis of aqueous bromide solutions to bromate, Electrochim. Acta, 1984, vol. 29, no. 7, p. 875.
Pavlovic, O.Z., Krstajić, N.V., and Spasojević, M.D., Formation of bromates at a RuO2/TiO2 titanium anode, Surf. Coatings Technol., 1988, vol. 34, no. 2, p. 177.
Conway, B.E., Phillips, Y., and Qian, S.Y., Surface electrochemistry and kinetics of anodic bromine formation at platinum, Journal of the Chemical Society, Faraday Trans., 1995, vol. 91, no. 2, p. 283.
Xu, J., Georgescu, N.S., and Scherson, D.A., The Oxidation of Bromide on Platinum Electrodes in Aqueous Acidic Solutions: Electrochemical and In Situ Spectroscopic Studies, J. Electrochem. Soc., 2014, vol. 161, no. 6, p. 392.
Johnson, D.C. and Bruckenstein, S., A Ring-Disk Study of HOBr Formation at Platinum Electrodes in 1.0 M H2SO4, J. Electrochem. Soc., 1970, vol. 117, no. 4, p. 460.
Grgur, B.N., Electrochemical Oxidation of Bromides on DSA/RuO2 Anode in the Semi-Industrial Batch Reactor for On-Site Water Disinfection, J. Electrochem. Soc., 2019, vol. 166, no. 2, p. 50.
Petrov, M.M., Loktionov, P.A., Konev, D.V., and Antipov, A.E., Evolution of Anolyte Composition in the Oxidative Electrolysis of Sodium Bromide in a Sulfuric Acid Medium, Russ. J. Electrochem., 2019, vol. 54, p. 1233.
Mastragostino, M. and Gramellini, C., Kinetic study of the electrochemical processes of the bromine/bromine aqueous system on vitreous carbon electrodes, Electrochim. Acta, 1985, vol. 30, no. 3, p. 373.
Ferro, S. and De Battisti, A., The bromine electrode. Part I: Adsorption phenomena at polycrystalline platinum electrodes, J. Appl. Electrochem., 2004, vol. 34, no. 10, p. 981.
Ferro, S., Orsan, C., and De Battisti, A., The bromine electrode. Part II: Reaction kinetics at polycrystalline Pt, J. Appl. Electrochem., 2005, vol. 35, no. 3, p. 273.
Ferro, S., The bromine electrode Part III: Reaction kinetics at highly boron-doped diamond electrodes, J. Appl. Electrochem., 2005, vol. 35, no. 3, p. 279.
Petrov, M.M., Konev, D.V., Kuznetsov, V.V., Antipov, A.E., Glazkov, A.T., and Vorotyntsev, M.A., Electrochemically driven evolution of Br-containing aqueous solution composition, J. Electroanal. Chem., 2019, vol. 836, p. 125.
Kelley, C.M. and Tartar, H.V., On the system: bromine-water, J. Amer. Chem. Soc., 2019, vol. 78, p. 5752.
Hill, J.O., Worsley, I.G., and Hepler, L.G., Calorimetric determination of the distribution coefficient and thermodynamic properties of bromine in water and carbon tetrachloride, J. Phys. Chem., 1968, vol. 72, p. 3695.
Kelsall, G.H., Welham, N.J., and Diaz, M.A., Thermodynamics of Cl–H2O, Br–H2O, I–H2O, Au–Cl–H2O, Au–Br–H2O and Au-I-H2O systems at 298 K, J. Electroanal. Chem., 1993, vol. 361, nos. 1–2, p. 13.
Beckwith, R.C., Wang, T.X., and Margerum, D.W., Equilibrium and kinetics of bromine hydrolysis, Inorg. Chem., 1996, vol. 35, p. 995.
Field, R.J. and Forsterling, H.-D., On the Oxybromine Chemistry Rate Constants with Cerium Ions in the Field-Koros-Noyes Mechanism of the Belousov–Zhabotinskii Reaction: The Equilibrium HBrO2 + \({\text{BrO}}_{{\text{3}}}^{ - }\) + H+ – \({\text{2BrO}}_{{\text{2}}}^{*}\) + H2O, J. Phys. Chem., 1986, vol. 90, no. 21, p. 5400.
Kshirsagar, G. and Field, R.J., A kinetic and thermodynamic study of component processes in the equilibrium 5HOBr = 2Br2 + \({\text{BrO}}_{{\text{3}}}^{ - }\) + 2H2O + H+, J. Phys. Chem., 1988, vol. 92, p. 7074.
Gyorgyi, L., Turanyi, T., and Field, R.J., Mechanistic details of the oscillatory Belousov–Zhabotinskii reaction, J. Phys. Chem., 1990, vol. 94, no. 18, p. 7162.
Liebhafsky, H.A., The equilibrium constant of the bromine hydrolysis and its variation with temperature, J. Amer. Chem. Soc., 1934, vol. 56, p. 1500.
Liebhafsky, H.A., The Hydrolysis of Bromine. The Hydration of the Halogens. The Mechanism of Certain Halogen Reactions, J. Amer. Chem. Soc, 1939, vol. 61, p. 3513.
Alves, W.A., Téllez, C.A., Sala, S.O., Santos, P.S., and Faria, R.B., Dissociation and rate of proton transfer of HXO3 (X = Cl, Br) in aqueous solution determined by Raman spectroscopy, J. Raman Spectroscopy, 2001, vol. 32, p. 1032.
Sáez, C., Sánchez-Carretero, A., Cañizares, P., and Rodrigo, M.A., Electrochemical synthesis of perbromate using conductive-diamond anodes, J. Appl. Electrochem., 2010, vol. 40, no. 10, p. 1715.
Tarasevich, M.R., Sadkowsky, A., and Yeager, E., Oxygen Electrochemistry, in: Comprehensive Treatise of Electrochemistry. New York: Plenum, 1983, p. 301.
Reier, T., Oezaslan, M., and Strasser P., Electrocatalytic Oxygen Evolution Reaction (OER) on Ru, Ir, and Pt Catalysts: A Comparative Study of Nanoparticles and Bulk Materials, ACS Catal., 2012, vol. 2, p. 1765.
Mussini, T. and Longhi, P., The Halogens. Bromine, in: Bard, A.J., Parsons, R., and Jordan, J. (Eds.), Standard Potentials in Aqueous Solutions, 1st ed., New York: Marcel Dekker, 1985, p. 78.
Sander, R., Compilation of Henry’s Law Constants for Inorganic and Organic Species of Potential Importance in Environmental Chemistry, http://www.mpchmainz. mpg.de/~sander/res/henry.html, Version 3, 1999, p. 10 (“bromine (Br)” section).
Funding
This work is carried out under the financial support of the Ministry of Sciences and Higher Education RF (project no. 14.574.21.0150, UIN RFMEFI57417X0150).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by Yu. Pleskov
In memory of the famous electrochemist Vladimir Sergeevich Bagotzky whose centenary is celebrated in 2020.
APPENDIX
APPENDIX
The systems compositions will be calculated, basing on thermodynamic equations (А1)–(А16) for the equilibrium electrochemical, chemical, and physical transformations listed in Table 2.
In these equations we use the following notation: Х for a Br-containing component in its aqueous solutions (Br–, Br2, \({\text{Br}}_{{\text{3}}}^{ - },\) \({\text{Br}}_{{\text{5}}}^{ - },\) HBrO, BrO–, \({\text{BrO}}_{{\text{3}}}^{ - },\) and \({\text{BrO}}_{{\text{4}}}^{ - }\)), {X} for its activity, \({\text{Br}}_{{\text{2}}}^{{{\text{liq}}}}\) and \({\text{Br}}_{{\text{2}}}^{{{\text{vap}}}}\) for the phases of liquid bromine or its vapor, \(\left\{ {{\text{Br}}_{{\text{2}}}^{{{\text{vap}}}}} \right\}\) for the bromine vapor activity (in molar concentration units: mol/dm3), Vsol and Vgas for the solution and gas phase volumes, \(E_{i}^{^\circ }\) for the standard potential of the corresponding electrochemical reaction, Ki for the equilibrium constant of the corresponding chemical transformation or for the transfer into gas phase (Kvap). The lower index “sat” denotes the system state in which the dissolved bromine and bromide-anion are equilibrated with the liquid bromine phase [equation (А7)], so that {Br2} = {Br2}sat = 0.185 М [21]; the activities of the rest components are determined by the corresponding thermodynamic equations, in particular, equation (А7) for {Br–}sat. The entry “log” in this work always means common (decimal) logarithm, in particular, in equations (А1)–(А16) and below. The parameter A = F/(RTln 10) = 16.92 V–1 (at room temperature).
For the components [Xi] concentrations all equations (А1)–(А16) hold when the components’ activities {Xi} are replaced by [Xi], the standard potential \(E_{i}^{^\circ }\) of each reaction is replaced by the apparent potential \(E_{i}^{{^\circ {\kern 1pt} '}},\) the transformation equilibrium constant Ki, by the apparent equilibrium constant \(K_{i}^{'}\) [18]. Further calculations were carried out under the assumption that the components’ activities in all expressions of Table 2 are replaced by their concentrations; in these equations, we used thermodynamic values of the parameters.
The Method for Determining of Concentrations of the System’s Components (described at length in works [13, 18])
The calculations are carried out for the known values of the solution рН and the solution:gas phase volume ratio (in these calculations we took the ratio V gas/V sol = 0.8) for one of the chosen values of the potential Е. First, we consider the system’s critical state, that is, such a summary concentration ctot at which the dissolved bromine concentration (approximately equal to its activity) reaches its value in saturated solution: {Br2}sat = 0.185 М, according to equation (А7), whereas the liquid bromine phase is still absent: \(\left[ {{\text{Br}}_{{\text{2}}}^{{{\text{liq}}}}} \right]\) = 0. Then equations (А1) or (А7), (А3), (А5), (А6), (А8), (А10), (А13), and (А14) allow calculating the critical concentrations \(c_{i}^{*}\) for all other components in the solution, and the effective concentration of bromine as a vapor above the solution. The summing of the concentrations for all components gives the critical value of the summary concentration \(c_{{{\text{tot}}}}^{*}.\) When the given value of the Br-atom summary concentration, that is, the bromide initial concentration ctot, exceeds its critical value: ctot > \(c_{{{\text{tot}}}}^{*},\) then concentrations of all the components (other than liquid bromine) are equal to their critical concentrations \(c_{i}^{*}\). And the liquid bromine effective concentration (its amount divided by the solution volume) is 1/2 (ctot – \(c_{{{\text{tot}}}}^{*}\)).
More complicated is the mathematical problem related to the case when the Br-atom summary concentration is below its critical value (at given parameters Е and рН): ctot < \(c_{{{\text{tot}}}}^{*}.\) Here the liquid bromine phase does not form, that is \(\left[ {{\text{Br}}_{{\text{2}}}^{{{\text{liq}}}}} \right]\) = 0. Then eight equilibrium interrelations between other components’ concentrations (А1), (А3), (А5), (А6), (А8), (А10), (А13), and (А14), combined with equation (14) for the known values of the summary concentration ctot, potential Е and рН in aggregate give a set of nine nonlinear algebraic equations for nine unknown components’ concentrations, see Table 1 (excluding \({\text{Br}}_{{\text{2}}}^{{{\text{liq}}}}\)). By using the method of sequential exclusion of the unknown variables, thise set can be reduced to a quintic equation with respect to the bromide-ion [Br–] concentration, or that of bromate-ion \(\left[ {{\text{BrO}}_{{\text{3}}}^{ - }} \right].\) The former one was used for the further solution, provided the potential Е was below the standard potential of the redox-couple Br–/\({\text{BrO}}_{{\text{3}}}^{ - },\) that is, E < \(E_{{\text{3}}}^{^\circ },\) the latter one, at higher potentials. In both cases, we applied the bisection method to a segment of possible values of the measured concentration accurate within 10–16. From the found solution, by using equation (13) we determined all concentrations, as well as the number of moles of each component Ni; by using equation (10), the summary redox-charge; by using equation (12), the average oxidation degree х.
The calculating of the bromine-atom summary concentration validated the above-discussed calculations’ correctness.
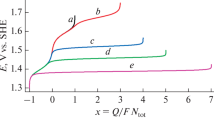
The performing of such calculations (at fixed values of рН and ctot) for a set of various potential values allows determining the dependence of х on Е (see Fig. 1), as well as the dependence of the system’s composition on the electrode potential Е (Fig. 3) or average oxidation degree х (Fig. 2).
Rights and permissions
About this article
Cite this article
Petrov, M.M., Konev, D.V., Antipov, A.E. et al. Theoretical Analysis of Changes in the System’s Composition in the Course of Oxidative Electrolysis of Bromide Solution: pH Dependence. Russ J Electrochem 56, 883–898 (2020). https://doi.org/10.1134/S1023193520100109
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193520100109