Abstract
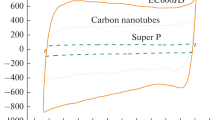
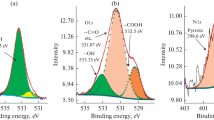
The necessity of the studying of carbonaceous materials differing in their surface area and structure is called for by the fact that these materials are used until now in the designing of positive electrodes for lithium-oxygen current sources. Under the model conditions, the effect of some factors on the effectiveness of oxygen reduction reaction at the positive electrode is studied. Among them are: properties of the dimethylsulfoxide- and acetonitrile-based electrolytes, the carbonaceous material (ХС 72, Super P, and carbon nanotubes) structure and its relevant transport processes depending on the electrode active layer mass (thickness) and the polarization current density, which determines the oxygen reaction effectiveness at the carbonaceous material. The electrochemically active surface area is shown to increase with the specific surface area, which is determined by the carbonaceous material porous structure, its mass at the electrode, the solvent properties, and the reaction rate. The active layer thickness and current density must be chosen for each carbonaceous material individually, depending upon its structure. At that, the active layer entire surface must be electrochemically accessible; it must make possible the lithium peroxide formation and subsequent decomposition. In the dimethylsulfoxide-based electrolyte (high donor number), the oxygen reduction reaction is highly reversible; the lithium peroxide formation here occurs via disproportionation in the solution bulk and results in the formation of Li2O2 particles with disordered (in all probability, toroidal) structure. This facilitates the back reaction (Li2O2 anodic decomposition), in good agreement with literature data [1]. In acetonitrile-based electrolyte (low donor number), the oxygen reduction reaction occurs in adsorbed state, producing LiО2 that disproportionates at the electrode surface forming a lithium peroxide insulating film whose oxidation needs high overvoltage. On the strength of all the parameters, carbon nanotubes are most effective in the oxygen reduction reaction in the dimethylsulfoxide-based electrolyte, because the carbon nanotubes have large volume of mesopores for the reactant transport, high electrochemically active surface area for the Li2O2 accumulation, and thus provide high characteristics per electrode.



Similar content being viewed by others
REFERENCES
Aetukuri, N.B., McCloskey, B.D., García, J.M., Krupp, L.E., Viswanathan, V., and Luntz, A.C., Solvating additives drive solution-mediated electrochemistry and enhance toroid growth in non-aqueous Li–O2 batteries, Nat. Chem., 2015, vol. 7, p. 50.
Xin Guo, Bing Sun, Dawei Su, Xiaoxue Liu, Hao Liu, Yong Wang, and Guoxiu Wang, Recent developments of aprotic lithium-oxygen batteries: functional materials determine the electrochemical performance, Science Bulletin, 2017, vol. 62, no. 6, p. 442.
Tan, P., Shyy, W., An, L., Wei, Z.H., and Zhao, T.S., A gradient porous cathode for non-aqueous lithiumair batteries leading to a high capacity, Electrochem. Commun., 2014, vol. 46, p. 111.
Ruimin Yu, Wugang Fan, Xiangxin Guo, and Shaoming Dong, Highly ordered and ultra-long carbon nanotube arrays as air cathodes for high-energy-efficiency Li-oxygen batteries, J. Power Sources, 2016, vol. 306, p. 402.
Jiang, H.R., Zhao, T.S., Shi, L., Tan, P., and An, L., First-Principles Study of Nitrogen-, Boron-Doped Graphene and Co-Doped Graphene as the Potential Catalysts in Nonaqueous Li–O2 Batteries, J. Phys. Chem. C., 2016, vol. 120, no. 12, p. 6612.
Wang, L., Ara, M., Wadumesthrige, K., Salley, S., and Simon, K.Y., Graphene nanosheet supported bifunctional catalyst for high cycle life Li-air batteries, J. Power Sources, 2013, vol. 234, p. 8.
Mitchell, R.R., Gallant, B.M., Thompson, C.V., and Shao-Horn, Y., All-carbon-nanofiber electrodes for high-energy rechargeable Li–O2 batteries, Energy Environ. Sci., 2011, vol. 4, p. 2952.
Zhang, S.S., Kang, Xu, and Read, J., A non-aqueous electrolyte for the operation of Li/air battery in ambient environment, J. Power Sources, 2011, vol. 196, p. 3906.
Galiotea, N.A., Jeong, S., Moraisa, W.G., Passerini, S., and Huguenina, F., The Role of Ionic Liquid in Oxygen Reduction Reaction for Lithium–air Batteries, Electrochim. Acta, 2017, vol. 247, p. 610.
Donghong, D., Xiu, You, Wenjun, Ren, Huikai, Wei, Huihong, Liu, and Shibin, Liu. Oxygen reduction reaction of different electrodes in dimethyl sulfoxide solvent for Li–air batteries, Intern. J. Hydrogen Energy, 2015, vol. 40, no. 34, p. 10847.
Feng, Wu, Yi, Xing, Li, L., Ji, Qian, Wenjie, Qu, Jianguo, Wen, Miller, D., Yusheng, Ye, Renjie, Chen, Amine, K., and Jun, Lu, Facile Synthesis of Boron-Doped rGO as Cathode Material for High Energy Li‒O2 Batteries, ACS Appl. Mater. Interfaces, 2016, vol. 8, no. 36, p. 23635.
Yue, Shen, Dan, Sun, Ling, Yu, Wang, Zhang, Yuanyuan, Shang, Huiru, Tang, Junfang, Wu, Anyuan, Cao, and Yunhui, Huang, A high-capacity lithium–air battery with Pd modified carbon nanotube sponge cathode working in regular air, Carbon, 2013, vol. 62, p. 288.
Cheng, H. and Scott, K., Carbon-supported manganese oxide nanocatalysts for rechargeable lithium–air batteries, J. Power Sources, 2010, vol. 195, no. 5, p. 1370.
Alaf, M., Tocoglu, U., Kartal, M., and Akbulut, H., Graphene supported heterogeneous catalysts for Li–O2 batteries, Applied Surf. Sci., 2016, vol. 380, p. 185.
Belova, A.I., Kwabi, D.G., Yashina, L.V., Shao-Horn, Y., and Itkis, D.M., Mechanism of Oxygen Reduction in Aprotic Li–Air Batteries: The Role of Carbon Electrode Surface Structure, J. Phys. Chem. C., 2017, vol. 121, no. 3, 1569.
Jinliang, Y., Jong-Sung, Y., and Sunden, B., Review on mechanisms and continuum models of multi-phase transport phenomena in porous structures of non-aqueous Li-Air batteries, J. Power Sources, 2015, vol. 278, p. 352.
Woo, H., Kang, J., Kim, J., Kim, C., Nam, S., and Park, B., Development of Carbon-Based Cathodes for Li–Air Batteries: Present and Future, Electron. Mater. Lett., 2016, vol. 12, no. 5, p. 551.
Tan, P., Wei, K., Zongping, S., Meilin, L., and Meng, N., Advances in modeling and simulation of Li‒air batteries, Progr. Energy Combustion Sci., 2017, vol. 62, p. 155.
Tan, P., Meng, Ni, Zongping, S., Bin, Chen, and Wei, Kong, Numerical investigation of a non-aqueous lithium-oxygen battery based on lithium superoxide as the discharge product, Applied Energy, 2017, vol. 203, p. 254.
Hardwick, L.J. and Bruce, P.G., The pursuit of rechargeable non-aqueous lithium–oxygen battery cathodes, Current Opinion/ Solid State Mater. Sci., 2012, vol. 16, no. 4, p. 178.
Ku-Bong, Chung, Ju-Kyung, Shin, Tae-Young, Jang, Dong-Kyun, Noh, Yongsug, Tak, and Sung-Hyeon, Baeck, Preparation and analyses of MnO2/carbon composites for rechargeable lithium–air battery, Rev. Adv. Mater. Sci., 2011, vol. 28, no. 1, p. 54.
Nitin, Kumar, Maxwell, D. Radin, B., Wood, C., Ogitsu, T., and Siegel, D.J., Surface-Mediated Solvent Decomposition in Li−Air Batteries: Impact of Peroxide and Superoxide Surface Terminations, J. Phys. Chem. C., 2015, vol. 119, no. 17, p. 9050.
Yuya, K., Yasushi, S., and Noriko, Yoshizawa, Contribution of mesopores in MgO-templated mesoporous carbons to capacitance in non-aqueous electrolytes, J. Power Sources, 2015, vol. 276, p. 176.
Wo, Xu, Kang, Xu, Viswanathan, V.V., Towne, S.A., Hardya, J.S., Jie, Xiaoa, Zimin, Niea, Dehong, Huc, Deyu, Wanga, and Ji-Guang, Zhanga, Reaction mechanisms for the limited reversibility of Li–O2 chemistry in organic carbonate electrolytes, J. Power Sources, 2011, vol. 196, p. 9631.
Laoire, C.O., Mukerjee, S., Abraham, K.M., Plichta, E.J., and Hendrickson M.A., Influence of Nonaqueous Solvents on the Electrochemistry of Oxygen in the Rechargeable Lithium-Air Battery, J. Phys. Chem. C., 2010, vol. 114, p. 9178.
Calvo, E.J. and Mozhzhukhina, N., A rotating ring disk electrode study of the oxygen reduction reaction in lithium containing nonaqueous electrolyte, Electrochem. Commun., 2013, vol. 31, p. 56.
Balaish, M., Kraytsberg, A., and Yair Ein-Eli, A critical review on lithium–air battery electrolytes, Phys. Chem. Chem. Phys., 2014, vol. 16, 2801.
Mozhzhukhina, N., Longinotti, M.P., Corti, H.R., and Calvo, E.J., A conductivity study of preferential solvation of lithium ion in acetonitrile-dimethyl sulfoxide mixtures, Electrochim. Acta, 2015, vol. 154, p. 456.
Bryantsev, V.S., Predicting the stability of aprotic solvents in Li-air batteries: pKa calculations of aliphatic C–H acids in dimethyl sulfoxide, Chem. Phys. Lett., 2013, vol. 558, p. 42.
Gritzner, G. and Kuta, J., Recommendations on reporting electrode potentials in nonaqueous solvents, Pure Appl.Chem., 1982, vol. 54, no. 8, p. 1527.
Meini, S., Piana, M., Beyer, H., Schwammlein, J., and Gasteiger, H.A., Effect of Carbon Surface Area on First Discharge Capacity of Li–O2 Cathodes and Cycle-Life Behavior in Ether-Based Electrolytes, J. Electrochem. Soc., 2012, vol. 159, p. A2135.
Gregg, S.J. and Sing, K.S.W., Adsorption, Specific Area, Porosity. Academic, 1982.
Dubinin M.M., Chemistry and Physics of Carbon, New York, Marcel Dekker, 1966. vol. 2, p. 51.
Volochshuk, A.M., Dubinin, M.M., Moskovskaya, T.A., Ivakhnyuk, G.K., and Fedorov, N.F., Izv. Akad. nauk SSSR, Ser. Khim., 1988, no. 2, p. 277.
Bogdanovskaya, V.A., Zhutaeva, G.V., Radina, M.V., Kazanskii, L.P., Tarasevich, M.R., Koltsova, E.M., Skichko, E.A., and Gavrilova, N.N., Physico-chemical properties of carbon nanotubes as supports for cathode catalysts of fuel cells. Surface structure and corrosion resistance, Prot. Metals Phys. Chem. Surf., 2016, vol. 52, no. 1, p. 45.
Xin, Guo, Bing, Sun, Dawei, Su, Xiaoxue, Liu, Hao, Liu, Yong, Wang, and Guoxiu, Wang, Recent developments of aprotic lithium-oxygen batteries: functional materials determine the electrochemical performance, Sci. Bulletin, 2017, vol. 62, p. 442.
Johnson, L., Li, C., Liu, Z., Chen, Y., Freunberger, S.A., Ashok, P.C., Praveen, B.B., Dholakia, K., Tarascon, J.M., and Bruce, P.G., The Role of LiO2 Solubility in O2 Reduction in Aprotic Solvents and Its Consequences for Li–O2 Batteries, Nat. Chem., 2014, vol. 6, no. 12, p. 1091.
Funding
This work was financed by the Ministry of sciences and higher education of RF and (in part) by the Russian foundation of basic research (project 16-03-00378).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by Yu. Pleskov
Rights and permissions
About this article
Cite this article
Bogdanovskaya, V.A., Panchenko, N.V., Radina, M.V. et al. Oxygen Reaction at Carbonaceous Materials with Different Structure in Electrolytes Based on Lithium Perchlorate and Aprotic Solvents. Russ J Electrochem 55, 878–888 (2019). https://doi.org/10.1134/S1023193519090040
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193519090040