Abstract
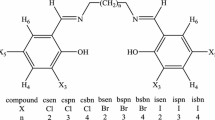
In the present work, the Schiff bases were synthesized by reacting sulfamethizole (SMTZ) with two different hydroxy benzaldehydes (2,3-dihydroxy benzaldehyde (DHBA) and 2,4,6-trihydroxy benzaldehyde (THBA)) and characterized by elemental analysis, 1H-NMR and IR spectroscopies. From the obtained data, it was suggested that 4,6-dihydroxy salicylaldehyde reacted with both primary and secondary amine groups of SMTZ. The binding properties between the synthesized Schiff bases and calf thymus DNA (CT-DNA) at the physiological pH (7.4) was investigated by using cyclic voltammetry and UV-Vis spectroscopy techniques. The experimental results verify that the Schiff bases can bind to CT-DNA by electrostatic mode in 1 : 1 stoichiometry. Antifungal activities of the synthesized Schiff bases against Candida albicans ATCC 10231 were studied and their minimum inhibitory concentrations (MIC) were also determined. The MIC value of the Schiff base 1 synthesized from DHBA is smaller than that of the Schiff base 2 obtained from THBA. Although Schiff base 2 binds to CT-DNA with a higher affinity than Schiff base 1, it is less effective than Schiff base 1 against Candida albicans.
Similar content being viewed by others
References
Khan, F., Khan, S., Athar, A., Ahmed, W., Zia-ul-Haq, and Khan, Z., Synthesis, spectral characterization and antibacterial study of a Schiff base metal complexes derived from N-[(E)-(5-chloro-2-hydroxyphenyl)methylidene]-4-nitrobenzenesulfonamide, Amer.- Eur. J. Agric. Environ. Sci., 2015, vol. 15, p. 216.
Kumar, G., Kumar, D., Singh, C.P., Kumar, A., and Rana, V.B., Synthesis, physical characterization and antimicrobial activity of trivalent metal Schiff base complexes, J. Serb. Chem. Soc., 2010, vol. 75, p. 629.
Xiao, Y.-J., Diao, Q.-C., Liang, Y.-H., and Zeng, K., Two novel Co(II) complexes with two different Schiff bases: inhibiting growth of human skin cancer cells, Braz. J. Med. Biol. Res., 2017, vol. 50, p. e6390.
Han, R., Sun, Y., Kang, C., Sun, H., and Wei, W., Amphiphilic dendritic nanomicelle-mediated co-delivery of 5-fluorouracil and doxorubicin for enhanced therapeutic efficacy, J. Drug Target., 2017, vol. 25, p. 140.
Sohrabi, N., Rasouli, N., and Kamkar, M., Synthesis, characterization and DNA interaction studies of (N,N′-bis(5-phenylazosalicylaldehyde)-ethylenediamine) cobalt(II) complex, Bull. Korean Chem. Soc., 2014, vol. 35, p. 2523.
Ni, Y., Lin, D., and Kokot, S., Synchronous fluorescence and UV-Vis spectrometric study of the competitive interaction of chlorpromazine hydrochloride and neutral red with DNA using chemometrics approaches, Talanta, 2005, vol. 65, p. 1295.
Temerk, Y. and Ibrahim, H., Electrochemical studies and spectroscopic investigations on the interaction of an anticancer drug flutamide with DNA and its analytical applications, J. Electroanal. Chem., 2015, vol. 736, p. 1.
Carter, M.T. and Bard, A.J., Voltammetric studies of the interaction of tris(1,10-phenanthroline)cobalt(III) with DNA, J. Am. Chem. Soc., 1987, vol. 109, p. 7528.
Ahmadi, F., Alizadeh, A.A., Bakhshandeh-Saraskanrood, F., Jafari, B., and Khodadadian, M., Experimental and computational approach to the rational monitoring of hydrogen-bonding interaction of 2-imidazoli-dinethione with DNA and guanine, Food Chem. Toxicol., 2010, vol. 48, p. 29.
Ahmadi, F. and Jafari, B., Voltammetry and spectroscopy study of in vitro interaction of fenitrothion with DNA, Electroanalysis, 2011, vol. 23, p. 675.
Cox, P.J., Psomas, G., and Bolos, C.A., Characterization and DNA-interaction studies of 1,1-dicyano-2,2-ethylene dithiolate Ni(II) mixed-ligand complexes with 2-amino-5-methyl thiazole, 2-amino-2-thiazoline and imidazole. Crystal structure of [Ni(i-MNT)(2a-5mt)2], Bioorg. Med. Chem., 2009, vol. 17, p. 6054.
Gao, F., Wang, Q., Zheng, M., Li, S., Chen, G., Jiao, K., and Gao, F., Electrochemical studies on the recognition of a ternary copper complex to single-stranded DNA and double-stranded DNA, Int. J. Electrochem. Sci, 2011, vol. 6, p. 1508.
Zhang, X., Li, M., Cui, Y., Zhao, J., Cui, Z., Li, Q., and Qu, K., Electrochemical behavior of calcein and the interaction between calcein and DNA, Electroanalysis, 2012, vol. 24, p. 1878.
https://pubchem.ncbi.nlm.nih.gov/compound/sulfamethizole#section=Pharmacology-and-Biochemistry. Accessed Feb. 20, 2018.
Petrikaitè, V., Tarasevičius, E., and Pavilonis, A., New thiazolidones-4 with sulfamethizole-2 substituent as potential antifungal and antimicrobial preparations, Biologija, 2007, vol. 53, p. 45.
Pehlivan, V, Biçer, E., Genç Bekiroğlu, Y., and Dege, N., Synthesis, in vitro antibacterial activity and calf thymus DNA binding of sulfamethizole-based Schiff bases, Abstract E-Book, 4th Int. Türk-Pak Conf. on Chemical Sciences (ITPCCS 2017), Konya, Oct. 26–28, 2017, PP017.
Omanović, D. and Branica, M., Automation ofvoltammetric measurements by polarographic analyser PAR 384B, Croat. Chem. Acta, 1998, vol. 71, p. 421.
Hajian, R., Ekhlasi, E., and Daneshvar, R., Spectroscopic and electrochemical studies on the interaction of epirubicin with fish sperm DNA, E-J. Chem., 2012, vol. 9, p. 1587.
Naik, T.R.R. and Naik, H.S.B., Electrochemical investigation of DNA binding on carbaldehyde oxime by cyclic voltammetry, Int. J. Electrochem. Sci., 2008, vol. 3, p. 409.
Babkina, S.S. and Ulakhovich, N.A., Complexing of heavy metals with DNA and new bioaffinity method of their determination based on amperometric DNA-based biosensor, Anal. Chem., 2005, vol. 77, p. 5678.
Shah, A., Zaheer, M., Qureshi, R., Akhter, Z., and Nazar, M.F., Voltammetric and spectroscopic investigations of 4-nitrophenylferrocene interacting with DNA, Spectrochim. Acta Part A, 2010, vol. 75, p. 1082.
Asghar, F., Badshah, A., Shah, A., Rauf, M.K., Ali, M.I., Tahir, M.N., Nosheen, E., Zia-ur-Rehman, and Qureshi, R., Synthesis, characterization and DNA binding studies of organoantimony(V) ferrocenyl benzoates, J. Organomet. Chem., 2012, vol. 717, p. 1.
Hua, D. and Chung, T.-S., Universal surface modification by aldehydes on polymeric membranes for isopropanol dehydration via pervaporation, J. Membrane Sci., 2015, vol. 492, p. 197.
Sprung, M.A., A summary of the reactions of aldehydes with amines, Chem. Rev., 1940, vol. 26, p. 297.
Huang, A. and Caro, J., Covalent post-functionalization of zeolitic imidazolate framework ZIF-90 membrane for enhanced hydrogen selectivity, Angew. Chem. Int. Ed., 2011, vol. 50, p. 4979.
Patil, M.P. and Sunoj, R.B., Insights on co-catalyst-promoted enamine formation between dimethylamine and propanal through ab initio and density functional theory study, J. Org. Chem., 2007, vol. 72, p. 8202.
Chatziefthimiou, S.D., Lazarou, Y.G., Hadjoudis, E., Dziembowska, T., and Mavridis, I.M., Keto forms of salicylaldehyde Schiff bases: structural and theoretical aspects, J. Phys. Chem. B, 2006, vol. 110, p. 23701.
Filarowski, A. and Majerz, I., AIM analysis of intramolecular hydrogen bonding in O-hydroxy aryl Schiff bases, J. Phys. Chem. A, 2008, vol. 112, p. 3119.
Filarowski, A., Intramolecular hydrogen bonding in o-hydroxyaryl Schiff bases, J. Phys. Org. Chem., 2005, vol. 18, p. 686.
Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 3rd ed., Wayne: Clinical and Laboratory Standards Institute, 2008. Approved Standard no. M27-S3.
Meti, M.D., Abbar, J.C., Nandibewoor, S.T., and Chimatadar, S.A., Voltammetric oxidation of carbenicillin and its electro analytical applications at gold electrode, Cogent Chem., 2016, vol. 2, p. 1. https://doi.org/10.1080/23312009.2016.1235459
Gosser, D.K., Cyclic Voltammetry:Simulation and Analysis of Reaction Mechanisms, New York: VCH, 1993, p. 43.
Pedrero, M., de Villena, F.J.M., Pingarron, J.M., and Polo, L.M., Determination of dinoseb by adsorptive stripping voltammetry, Electroanalysis, 1991, vol. 3, p. 419.
Giannakopoulos, E., Deligiannakis, Y., and Salahas, G., Electrochemical interfacial adsorption mechanism of polyphenolic molecules onto Hanging Mercury Drop Electrode surface (HMDE), J. Electroanal. Chem., 2012, vol. 664, p. 117.
Coşkun, E. and Biçer, E., Sülfatiazolün Ni(II) iyonlariyla etkileşiminin voltametrik incelenmesi, Erciyes Univ. J. Inst. Sci. Technol., 2014, vol. 30, p. 296.
Sabry, S.M., Barary, M.H., Abdel-Hay, M.H., and Belal, T.S., Adsorptive stripping voltammetric behaviour of azomethine group in pyrimidine-containing drugs, J. Pharm. Biomed. Anal., 2004, vol. 34, p. 509.
Habib, I.H.I., Weshahyi, S.A., Toubar, S., and El-Alamin, M.M.A., Cathodic stripping voltammetric determination of losartan in bulk and pharmaceutical products, Portug. Electrochim. Acta, 2008, vol. 26, p. 315.
Manousek, O., Exner, O., and Zuman, P., Fission of activated carbon-nitrogen and carbon-sulphur bonds. XI. Polarographic reduction of substituted benzene-sulphoamides, in Topics on Organic Polarography, Zuman, P., Ed., London: Plenum Press, 1970, p. 322.
Chambers, J.Q., Organic sulphur compounds, in Encyclopedia of Electrochemistry of the Elements, Bard, A.J. and Lund, H., Eds., vol. XII: Organic Section, New York: M. Dekker, 1979, chapter XII-3, p. 371.
Smyth, M.R. and Smyth, W.F., Voltammetric methods for the determination of foreign organic compounds of biological significance, Analyst, 1978, vol. 103, p. 529.
Çakir, S. and Biçer, E., Synthesis, spectroscopic and electrochemical characteristics of a novel Schiff-base from saccharin and tryptophan, J. Iran. Chem. Soc., 2010, vol. 7, p. 394.
Carter, M.T., Rodriguez, M., and Bard, A.J., Voltam-metric studies of the interaction of metal chelates with DNA. 2. Tris-chelated complexes of cobalt(III) and iron(II) with 1,10-phenanthroline and 2,2′-bipyridine, J. Am. Chem. Soc., 1989, vol. 111, p. 8901.
Tung, Y.-L., Lee, S.-W., Chi, Y., Chen, L.-S., Shu, C.-F., Wu, F.-I., Carty, A.J., Chou, P.-T., Peng, S.-M., and Lee, G.-H., Organic light-emitting diodes based on charge-neutral RuII phosphorescent emitters, Adv. Mater., 2005, vol. 17, p. 1059.
Pakravan, P. and Masoudian, S., Study on the interaction between isatin-ß-thiosemicarbazone and calf thymus DNA by spectroscopic techniques, Iran. J. Pharm. Res., 2015, vol. 14, p. 111.
Mallappa, M., Gowda, B.G., and Mahesh, R.T., Mechanism of interaction of antibacterial drug moxifloxacin with herring sperm DNA: electrochemical and spectroscopic studies, Der Pharma Chem., 2014, vol. 6, p. 398.
Jiang, Y., Yuan, Y., Wang, K., Li, H., Xu, C., and Yang, X., Spectroscopic and electrochemical studies on the binding of pyrocatechol violet with telomere DNA and its application, Int. J. Electrochem. Sci., 2012, vol. 7, p. 10933.
Acknowledgments
This study was presented in part and in poster form at International Eurasian Conference on Biological and Chemical Sciences (EurasianBioChem 2018), 26–27 April 2018, Ankara/TURKEY.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Elektrokhimiya, 2019, Vol. 55, No. 5, pp. 587–598.
The article is published in the original.
Rights and permissions
About this article
Cite this article
Biçer, E., Pehlivan, V. & Bekiroğlu, Y.G. Synthesis, Characterization, in vitro Antifungal Activities and Calf Thymus DNA Interactions of Two Different Hydroxy Benzaldehyde Derivative Schiff Bases from Sulfamethizole: Electrochemical, Spectroscopic and Biological Study. Russ J Electrochem 55, 419–428 (2019). https://doi.org/10.1134/S1023193519050045
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193519050045