Abstract
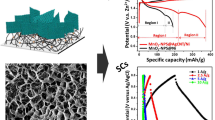
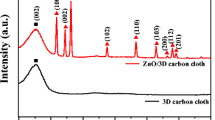
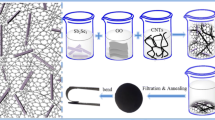
For the first time, the positive carbon rod of zinc-carbon battery (battery carbon rod electrode, BCRE) was used as a new working electrode and its electrochemical behavior was compared with carbon paste and glassy carbon electrodes in KCl solution containing Fe(CN6)3–/4– ions as probe agent. Then, the sponge/raspberry-like Au nanoclusters (AuNCs) were synthesized on BCRE by one-step electrodeposition of HAuCl4 in phosphate and nitrate buffer solution and the electrochemical properties of surfaces was investigated in probe media and sulfuric acid. This fabrication method was simple, facile and controllable, without any seed, template or surfactant.
Similar content being viewed by others
References
Sepeur, S., Nanotechnology Technical Basics and Applications, Vincentz, Hannover, 2008.
Seo, B., Choi, S., and Kim, J., Simple electrochemical deposition of Au nanoplates from Au (I) cyanide complexes and their electrocatalytic activities, ACS Appl. Mater. Interfaces, 2011, vol. 3, p.441.
Li, F., Han, X., and Liu, S., Development of an electrochemical DNA biosensor with a high sensitivity of fM by dendritic gold nanostructure modified electrode, Biosens. Bioelectron, 2011, vol. 26, p. 2619.
Ye, W., Yan, J., Ye, Q., and Zhou, F., Template-free and direct electrochemical deposition of hierarchical dendritic gold microstructures: growth and their multiple applications, J. Phys. Chem. C, 2010, vol. 114, p. 15617.
Xu, X., Jia, J., Yang, X., and Dong, S., A templateless, surfactantless, simple electrochemical route to a dendritic gold nanostructure and its application to oxygen reduction, Langmuir, 2010, vol. 26, p. 7627.
Huang, T., Meng, F., and Qi, L., Controlled synthesis of dendritic gold nanostructures assisted by supramolecular complexes of surfactant with cyclodextrin, Langmuir, 2009, vol. 26, p. 7582.
Zhou, D.L., Wang, R.Z., Zhang, M., Weng, X., Chen, J.R., Wang, A.J., and Feng, J.J., Iron(III) ionsupported electrosynthesis of urchin-like gold arrays, Electrochim. Acta, 2013, vol. 108, p.390.
Guo, S. and Wang, E., Synthesis and electrochemical applications of gold nanoparticles, Anal. Chim. Acta, 2007, vol. 598, p.181.
Khoury, C.G. and Vo-Dinh, T., Gold nanostars for surface-enhanced Raman scattering: Synthesis, characterization and optimization, J. Phys. Chem. C, 2008, vol. 112, p. 18849.
Jana, N.R., Gearheart, L., and Murphy, C.J., Wet chemical synthesis of high aspect ratio cylindrical gold nanorods, J. Phys. Chem. B, 2001, vol. 105, p. 4065.
Das, A.K. and Raj, C.R., Rapid room temperature synthesis of electro catalytically active Au nanostructures, J. Colloid Interface. Sci., 2011, vol. 353, p.506.
Jena, B.K. and Raj, C.R., Shape-controlled synthesis of gold nanoprism and nanoperiwinkles with pronounced electrocatalytic activity, J. Phys. Chem. C, 2007, vol. 111, p. 15146.
Jena, B.K. and Raj, C.R., Synthesis of flower-like gold nanoparticles and their electrocatalytic activity towards the oxidation of methanol and the reduction of oxygen, Langmuir, 2007, vol. 23, p. 4064.
Vasilev, K., Zhu, T., Wilms, M., Gillies, G., Lieberwirth, I., Mittler, S., Knoll, W., and Kreiter, M., Simple, one-step synthesis of gold nanowires in aqueous solution, Langmuir, 2005, vol. 21, p. 12399.
Huang, C.J., Wang, Y.H., Chiu, P.H., Shih, M.C., and Meen, T.H., Electrochemical synthesis of gold nanocubes, Mater. Lett., 2006, vol. 60, p. 1896.
Chen, S., Wang, Z.L., Ballato, J., Foulger, S.H., and Carroll, D.L., Monopod, bipod, tripod and tetrapod gold nanocrystals, J. Am. Chem. Soc., 2003, vol. 125, p. 16186.
Li, C., Shuford, K.L., Chen, M., Je Lee, E., and Cho, S.O., A facile polyol route to uniform gold octahedra with tailorable size and their optical properties, ACS Nano, 2008, vol. 2, p. 1760.
Wu, H.L., Chen, C.H., and Huang, M.H., Seed-mediated synthesis of branched gold nanocrystals derived from the side growth of pentagonal Bi pyramids and the formation of gold nanostars, Chem. Mater., 2009, vol. 21, p.110.
Seo, B., Choi, S., and Kim, J., Simple electrochemical deposition of Au nanoplates from Au(I) cyanide complexes and their electrocatalytic activities, ACS Appl. Mater. Interfaces, 2011, vol. 3, p.441.
Qin, Y., Song, Y., Sun, N., Zhao, N., Li, M., and Qi, L., Ionic liquid-assisted growth of single-crystalline dendritic gold nanostructures with a three-fold symmetry, Chem. Mater., 2008, vol. 20, p. 3965.
Das, A.K. and Raj, C.R., Iodide-mediated reduction of AuCl4 and a new green route for the synthesis of single crystalline Au nanostructures with pronounced electrocatalytic activity, J. Phys. Chem. C, 2011, vol. 115, p. 21041.
Lu, G., Li, C., and Shi, G., Synthesis and characterization of 3D dendritic gold nanostructures and their use as substrates for surface-enhanced Raman scattering, Chem. Mater., 2007, vol. 19, p. 3433.
Duan, G., Cai, W., Luo, Y., Li, Z., and Lei, Y., Electrochemically induced flowerlike gold nanoarchitectures and their strong surface-enhanced Raman scattering effect, Appl. Phys. Lett., 2006, vol. 89, p. 211905.
Li, Y. and Shi, G., Electrochemical growth of twodimensional gold nanostructures on a thin polypyrrole film modified ITO electrode, J. Phys. Chem. B, 2005, vol. 109, p. 23787.
Praig, V.G., Piret, G., Manesse, M., Castel, X., Boukherroub, R., and Szunerits, S., Seed-mediated electrochemical growth of gold nanostructures on indium tin oxide thin films, Electrochim. Acta, 2008, vol. 53, p. 7838.
Guo, S., Wang, L., and Wang, E., Templateless, surfactantless, simple electrochemical route to rapid synthesis of diameter-controlled 3D flowerlike gold microstructure with “clean” surface, Chem. Commun., 2007, vol. 30, p. 3163.
Yang, Y.C., Huang, T.K., Chen, Y.L., Mevellec, J.Y., Lefrant, S., Lee, C.Y., and Chiu, H.T., Electrochemical growth of gold nanostructures for surface-enhanced Raman scattering, J. Phys. Chem. C, 2011, vol. 115, p. 1932.
Wang, J., Duan, G., Liu, G., Li, Y., Dai, Z., Zhang, H., and Cai, W., Gold quasi rod-shaped nanoparticle-built hierarchically micro/nanostructured pore array via clean electrodeposition on a colloidal monolayer and its structurally enhanced SERS performance, J. Mater. Chem., 2011, vol. 21, p. 8816.
Chen, H., Kannan, P., Guo, L., Chen, H., and Kim, D.H., Direct growth of highly branched crystalline Au nanostructures on an electrode surface: their surface enhanced Raman scattering and electrocatalytic applications, J. Mater. Chem., 2011, vol. 21, p. 18271.
Huang, C.J., Chiu, P.H., Wang, Y.H., Chen, W.R., and Mee, T.H., Synthesis of the gold nanocubes by electrochemical technique, J. Electrochem. Soc., 2006, vol. 153, p. D129.
Yu, Y.Y., Chang, S.S., Lee, C.L., and Wang, C.R.C., Gold nanorods: Electrochemical synthesis and optical properties, J. Phys. Chem. B, 1997, vol. 101, p. 6661.
Huan, T.N., Ganesh, T., Kim, K.S., Kim, S., Han, S.H., and Chung, H., A three-dimensional gold nanodendrite network porous structure and its application for an electrochemical sensing, Biosens. Bioelectron, 2011, vol. 27, p.183.
O’Mullane, A.P., Ippolito, S.J., Sabri, Y.M., Bansal, V., and Bhargava, K., Premonolayer oxidation of nanostructured gold: An important factor influencing electrocatalytic activity, Langmuir, 2009, vol. 25, p. 3845.
Lin, T.H., Lin, C.W., Liu, H.H., Sheu, J.T., and Hung, W.H., Potential-controlled electrodeposition of gold dendrites in the presence of cysteine, Chem. Commun., 2011, vol. 47, p. 2044.
Feng, J.J., Li, A.Q., Lei, Z., and Wang, A.J., Lowpotential synthesis of “Clean” Au nanodendrites and their high performance toward ethanol oxidation, ACS Appl. Mater. Interfaces, 2012, vol. 4, p. 2570.
Feng, J.J., Lv, Z.Y., Qin, S.F., Li, A.Q., Fei, Y., and Wang, A.J., N-methylimidazole-assisted electrodeposition of Au porous textile-like sheet arrays and its application to electrocatalysis, Electrochim. Acta, 2013, vol. 102, p.312.
Lv, Z.Y., Li, A.Q., Fei, Y., Li, Z., Chen, J.R., Wang, A.J., and Feng, J.J., Facile and controlled electrochemical route to three-dimensional hierarchical dendritic gold nanostructures, Electrochim. Acta, 2013, vol. 109, p.136.
Zhou, D.L., Wang, R.Z., Zhang, M., Weng, X., Chen, J.R., Wang, A.J., and Feng, J.J., Iron(III) ionsupported electrosynthesis of urchin-like gold arrays, Electrochim. Acta, 2013, vol. 108, p.390.
Shiigi, H., Yamamoto, Y., Yoshi, N., Nakao, H., and Nagaoka, T., One-step preparation of positivelycharged gold nanoraspberry, Chem. Commun. 2006, vol. 41, p. 4288.
Manivannan, S. and Ramaraj, R., Electrodeposited nanostructured raspberry-like gold-modified electrodes for electrocatalytic applications, J. Nanopart. Res., 2013, vol. 15, p.1.
Takamura, T., Encyclopedia of Electrochemical Power Sources, 1st ed., Elsevier, 2010.
Cheng, T.M., Huang, T.K., Lin, H.K., Tung, S.P., Chen, Y.L., Lee, C.Y., and Chiu, H.T., (110)-Exposed gold nanocoral electrode as low onset potential selective glucose sensor, ACS Appl. Mater. Interfaces, 2010, vol. 2, p. 2773.
Ye, W., Kou, H., Liu, Q., Yan, J., Zhou, F., and Wang, C., Electrochemical deposition of Au–Pt alloy particles with cauliflower-like microstructures for electrocatalytic methanol oxidation, Int. J. Hydrogen Energy, 2012, vol. 37, p. 4088.
Ma, Y., Di, J., Yan, X., Zhao, M., Lu, Z., and Tu, Y., Direct electrodeposition of gold nanoparticles on indium tin oxide surface and its application, Biosens. Bioelectron, 2009, vol. 24, p. 1480.
Ye, W.C., Yan, J.F., Ye, Q., and Zhou, F., Templatefree and direct electrochemical deposition of hierarchical dendritic gold microstructures: Growth and their multiple applications, J. Phys. Chem. C, 2010, vol. 114, p. 15617.
Hu, Y., Jin, J., Wu, P., Zhang, H., and Cai, C., Graphene-gold nanostructure composites fabricated by electrodeposition and their electrocatalytic activity toward the oxygen reduction and glucose oxidation, Electrochim. Acta, 2010, vol. 56, p. 491.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Elektrokhimiya, 2018, Vol. 54, No. 8, pp. 723–730.
The article is published in the original.
Rights and permissions
About this article
Cite this article
Motaghedifard, M., Behpour, M. & Amani, A.M. Electrochemical Growth of Sponge/Raspberry-Like Gold Nanoclusters at the Carbon Rod. Russ J Electrochem 54, 629–635 (2018). https://doi.org/10.1134/S1023193518080037
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193518080037