Abstract
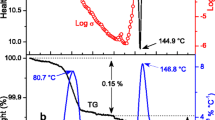
The Cs2HPO4 · 2H2O single crystals synthesized from an aqueous solution containing equimolar amounts of H3PO4 and Cs2CO3 were studied by impedance and IR spectroscopy, X-ray diffraction analysis, and differential scanning calorimetry (DSC). The IR spectra were analyzed in accordance with the structural data, and the absorption bands were assigned. The proton conductivity was studied at temperatures in the range 20–250°C. The conductivity of dehydrated Cs2HPO4 was low, ~10–5–10–9 S cm–1 at 90–250°C with an activation energy of conductivity E a = 1.1 eV at 130–250°C. The processes determining the character of the temperature dependence of conductivity were consistent with the DSC and thermogravimetry data. According to these data, dehydration of the crystalline hydrate Cs2HPO4 · 2H2O starts at 60°C and occurs in three stages, forming Cs2HPO4 · 1.5H2O below 100°C; anhydrous Cs2HPO4 at t > 160°C, which is stable up to 300°C; and Cs4P2O7 above 330°C.
Similar content being viewed by others
References
Busch, G. and Scherrer, P., Naturwissenshaften, 1935, vol. 23, p. 737.
Frazer, B.C. and Pepinsky, R., Acta Crystallogr., 1953, vol. 6, p. 273.
Reese, W. and May, L.F., Phys. Rev., 1967, vol. 162, p. 510.
Busch, G., Ferroelectrics, 1987, vol. 71, p. 17.
Baranov, A.I., Shuvalov, L.A., and Shchagina, N.M., Pis’ma Zh. Eksp. Teor. Fiz., 1982, vol. 36, p. 381.
Plakida, N.M., Phys. Status Solidi B, 1986, vol. 135, p. 133.
Uesu, Y. and Kobayashi, J., Phys. Status Solidi A, 1976, vol. 34, p. 475.
Matsunaga, H. and Itoh, K., J. Phys. Soc. Jpn., 1980, vol. 486, p. 2011.
Baranov, A.I., Khiznichenko, V.P., Sandler, V.A., and Shuvalov, L.A., Ferroelectrics, 1988, vol. 81, p. 1147.
Preisinger, A., Mereiter, K., and Bronowska, W., Mater. Sci. Forum, 1994, vol. 166, p. 511.
Boysen, D.A., Uda, T., Chisholm, C.R.I., and Haile, S.M., Science, 2004, vol. 303, p. 68.
Uda, T. and Haile, S.M., Electrochem. Solid-State Lett., 2005, vol. 8, p. A245.
Chisholm, C.R.I., Boysen, D.A., Papandrew, A.B., Zecevic, S., Cha, S.Y., Sasaki, K.A., Varga, A., Giapis, K.P., and Haile, S.M., Electrochem. Soc. Interface, 2009, vol. 18, p. 53.
Papandrew, A.B., Chisholm, C.R.I., Elgammal, R.A., Ozer, M.M., and Zecevic, S.K., Chem. Mater., 2011, vol. 23, p. 1659.
Nirsha, B., Gudinitsa, E.N., Efremov, V.A., and Fakeev, A.A., Russ. J. Inorg. Chem., 1983, vol. 28, p. 475.
Baur, W.H. and Khan, A.A., Acta Crystallogr., 1970, vol. B26, p. 1584.
Catti, M., Ferraris, G., and Franchini-Angela, M., Acta Crystallogr., 1977, vol. B33, p. 3449.
Ferraris, G., Jones, D.W., and Yerkess, J., Acta Crystallogr., 1971, vol. B27, p. 354.
Baran, J., Lis, T., and Ratajczak, H., J. Mol. Struct., 1989, vol. 195, p. 159.
Sheludyakova, L.A., Afanasieva, V.A., Podberezskaya, N.V., and Mironov, Yu.I., Russ. J. Struct. Chem., 1999, vol. 40, p. 869.
Stoger, B., Weil, M., and Zobetz, E., Z. Kristallogr., 2012, vol. 227, p. 859.
Stöger, B. and Weil, M., Acta Crystallogr., Sect. C: Struct. Chem., 2014, vol. 70, p. 7.
Lavrova, G.V., Bulina, N.V., Min’kov, V.S., and Matvienko, A.A., Russ. J. Inorg. Chem., 2016, vol. 61, p. 284.
Lavrova, G.V., Ponomareva, V.G., and Martsinkevich, V.V., Abstracts of Papers, 16 Int. Conf. “Solid State Protonic Conductors” (SSPC16), Grenoble, France, 2012, p. 123.
Ponomareva, V.G. and Lavrova, G.V., Abstracts of Papers, II Vseros. konf. “Goryachie tochki khimii tverdogo tela: mekhanizmy tverdofaznykh reaktsii” (II Russian Conf. “Hot Issues in Solid State Chemistry: Mechanisms of Solid-Phase Reactions”), Novosibirsk, 2015, p. 83.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.G. Ponomareva, I.N. Bagryantseva, G.V. Lavrova, 2017, published in Elektrokhimiya, 2017, Vol. 53, No. 6, pp. 715–720.
Rights and permissions
About this article
Cite this article
Ponomareva, V.G., Bagryantseva, I.N. & Lavrova, G.V. Proton conductivity and spectral data of Cs2HPO4 · 2H2O. Russ J Electrochem 53, 636–640 (2017). https://doi.org/10.1134/S1023193517060155
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193517060155