Abstract
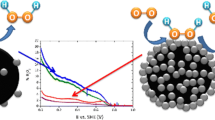
Electrochemical experiments with a rotating disk electrode are used to measure specific catalytic activity of Pt/C structures in the oxygen reduction reaction at the density of Pt nanoparticles on the glassy carbon support surface below one monolayer. The specific activity maximum is found at the coverage of about 0.4 monolayer. An explanation of the observed dependence is suggested that is based on consideration of the relationship between the surface density and charge state of the system of metallic catalyst particles. A numeric model is developed that describes charge transfer in the catalyst structure due to the difference in the work functions between the metal nanoparticles and support with account for the discrete nature of the nanoparticle charging and their mutual polarization. Calculations show that the carbon support coverage by Pt particles of about 0.4 monolayer corresponds to the largest amount of charged particles with the maximum energy of electrons, which provides the maximum catalyst activity and explains the dependence observed in the experiment.
Similar content being viewed by others
References
Revankar, S.T. and Majumdar, P., Fuel Cells: Principles, Design, and Analysis, CRC Press, 2014.
Ferreira, R.B., Falcao, D.S., Oliveira, V.B., and Pinto, A.M.F.R., J. Power Sources, 2015, vol. 277, p. 329.
Shao, M., Chang, Q., Dodelet, J.-P., and Chenitz, R., Chem. Rev., 2016, vol. 116, p. 3594.
Greeley, J., Stephens, I.E.L., Bondarenko, A.S., Johansson, T.P., Hansen, H.A., Jaramillo, T.F., Rossmeisl, J., Chorkendorff, I., and Nørskov, J.K., Nature Chem., 2009, vol. 1, p. 552.
Kuttiyiel, K.A., Choi, Y.M., Hwang, S.-M., Park G.-G., Yang, T.-H., Su, D., Sasaki, K., Liu, P., and Adzic, R.R., Nano Energy, 2015, vol. 13, p. 442.
Chen, C., Kang, Y., Huo, Z., Zhu, Z., Huang, W., Xin, H.L., Snyder, J.D., Li, D., Herron, J.A., Mavrikakis, M., Chi, M., More, K.L., Li, Y., Markovic, N.M., Somorjai, G.A., Yang, P., and Stamenkovic, V.R., Science, 2014, vol. 343, p. 1339.
Stephens, I.E.L., Bondarenko, A.S., Grønbjerg, U., Rossmeisl, J., and Chorkendorff, I., Energy Environ. Sci., 2012, vol. 5, p. 6744.
Fuhrmann, J., Zhao, H., Langmach, H., Seidel, Y.E., Jusys, Z., and Behm, R.J., Fuel Cells, 2011, vol. 11, p. 501.
Nesselberger, M., Roefzaad, M., Hamou, R.F., Biedermann, P.U., Schweinberger, F.F., Kunz, S., Schloegl, K., Wiberg, G.K.H., Ashton, S., Heiz, U., Mayrhofer, K.J.J., and Arenz, M., Nat. Mater., 2013, vol. 12, p. 919.
Karlberg, G.S., Rossmeisl, J., and Nørskov, J.K., Phys. Chem. Chem. Phys., 2007, vol. 9, p. 5158.
Kozhevin, V.M., Yavsin, D.A., Kouznetsov, V.M., Busov, V.M., Mikushkin, V.M., Nikonov, S.Yu., Gurevich, S.A., and Kolobov, A., J. Vac. Sci. Technol., B, 2000, vol. 18, p. 1402.
Grigoriev, A.I. and Shiriaeva, S.O., J. Phys. D: Appl. Phys., 1990, vol. 23, p. 1361.
Rostovshchikova, T.N., Smirnov, V.V., Kozhevin, V.M., Yavsin, D.A., Zabelin, M.A., Yassievich, I.N., and Gurevich, S.A., Appl. Catal., A, 2005, vol. 296, p. 70.
Gurevich, S.A., Kozhevin, V.M., Yassievich, I.N., Yavsin, D.A., Rostovshchikova, T.N., and Smirnov, V.V., Thin Films and Nanostructures, Physic-Chemical Phenomena in Thin Films and at Solid Surfaces, Amsterdam: Elsevier, 2007, vol. 34.
Zhang, Y., Pluchery, O., Caillard, L., Lamic-Humblot, A.-F., Casale, S., Chabal, Y.J., and Salmeron, M., Langmuir, 2013, vol. 29, p. 1634.
Rostovshchikova, T.N., Lokteva, E.S., Nikolaev, S.A., Golubina, E.V., Gurevich, S.A., Kozhevin, V.M., Yavsin, D.A., and Lunin, V.V., Catalysis: Principles, Types and Applications, New York Nova Sci. Publ., 2011.
Abeles, B., Sheng, P., Coutts, M.D., and Arie, Y., Adv. Phys., 1975, vol. 24, p. 407.
Il’yushchenkov, D.S., Kozhevin, V.M., and Gurevich, S.A., Fiz. Tverd. Tela, 2015, vol. 57, p. 1670.
Fomenko, V.S., Handbook of Thermionic Properties, New York Plenum Press, 1966.
David, G., Hall, D.G., and Cole, R.H., J. Phys. Chem., 1981, vol. 85, p. 1065.
Bennet, A.J. and Duke, C.B., Phys. Rev., 1967, vol. 160, p. 541.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © S.A. Gurevich, D.S. Il’yushchenkov, D.A. Yavsin, N.V. Glebova, A.A. Nechitailov, N.K. Zelenina, A.A. Tomasov, 2017, published in Elektrokhimiya, 2017, Vol. 53, No. 6, pp. 642–650.
Rights and permissions
About this article
Cite this article
Gurevich, S.A., Il’yushchenkov, D.S., Yavsin, D.A. et al. Charge state and activity of Pt/C catalysts in oxygen reduction reaction. Russ J Electrochem 53, 567–574 (2017). https://doi.org/10.1134/S1023193517060052
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193517060052