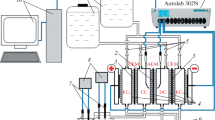
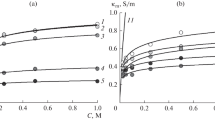
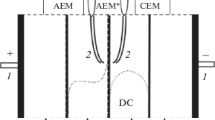
Abstract
The dynamics of changes in overall and partial voltammetric characteristics with respect to chloride and hydroxide ions is studied by the method of rotating membrane disk (RMD) under the conditions of stabilized diffusion layer thickness for the original strongly basic MA-41P and homogeneous AMX membranes and also for the modified heterogeneous MA-41P-M membrane at high current densities. For unmodified anion-exchange membranes at currents exceeding the limiting value, the hydrolysis of fixed ammonium bases produces secondary and ternary amino groups which are catalytically active in the reaction of water molecule dissociation. The hydrolysis of amino groups in the membrane surface layer is the mechanism of degradation of electrochemical characteristics of strongly basic membranes. This results in the increase of transport numbers with respect to hydroxide ions and weakening of mass transfer with respect to salt ions. For the surface-modified heterogeneous anion-exchange membranes, no degradation of electrochemical characteristics is observed. The characteristics of the surface-modified MA-41P-M membrane remain stable: after long-term operation of the energized membrane, the partial currents with respect to hydroxide ions are close to zero and the mass transfer with respect to salt ions is considerably intensified. The dependences of the thickness of the hydrolyzed layer of a strongly basic anion-exchange membrane on the time of its exposure to solutions of high pH are determined. An original method is developed for determination of the hydrolyzed layer thickness for strongly-basic anion-exchange membranes, which is based on the copper ability to form stable complex compounds with weakly basic amino groups of anion-exchange membranes.
Similar content being viewed by others
References
Sata, T., Tsujimoto, M., Yamaguchi, T., and Matsusaki, K., J. Membr. Sci., 1996, vol. 112, p. 161.
Hwang, U. and Choi, J.-H., Sep. Purif. Technol., 2006, vol. 48, p. 16.
Zabolotskii, V.I., Bugakov, V.V., Sharafan, M.V., and Chermit, R.Kh., Russ. J. Electrochem., 2012, vol. 48, p. 650.
Kononov, Yu.A. and Vrevskii, B.M., Zh. Prikl. Khim., 1971, vol. 44, p. 929.
Varentsov, V.K. and Pevnitskaya, M.V., Izv. Sib. Otd. Akad. Nauk SSSR, Ser. Khim. Nauk, 1973, no. 4, p. 134.
Gavish, B. and Lifson, S., J. Chem. Soc., Faraday Trans. 1, 1979, vol. 75, p. 463.
Tanaka, Y. and Seno, M., Denki Kagaku, 1983, vol. 51, p. 267.
Shaposhnik, V.A., Kastyuchik, A.S., and Kozaderova, O.A., Russ. J. Electrochem., 2008, vol. 44, p. 1073.
Zabolotskii, V.I., Shel’deshov, N.V., and Gnusin, N.P., Usp. Khim., 1988, vol. 57, p. 1403.
Zabolotskii, V.I., Nikonenko, V.V., Urtenov, M.Kh., Lebedev, K.A., and Bugakov, V.V., Russ. J. Electrochem., 2012, vol. 48, p. 692.
Zabolotskii, V.I., Loza, S.A., and Sharafan, M.V., Russ. J. Electrochem., 2005, vol. 41, p. 1053.
Zabolotskii, V.I. and Nikonenko, V.V., Perenos ionov v membranakh (Ion Transfer in Membranes), Moscow: Nauka, 1996.
Ghalloussi, R., Garcia-Vasquez, W., Bellakhal, N., Larchet, C., Dammak, L., Huguet, P., and Grande, D., Sep. Purif. Technol., 2011, vol. 80, p. 270.
Iwai, Y. and Yamanishi, T., Polym. Degrad. Stab., 2009, vol. V. 94. P. 679.
Cheng, Ch. and Fuller, T.F., Polym. Degrad. Stab., 2009, vol. 94, p. 1436.
Zabolotskii, V.I., Fedotov, Yu.A., Nikonenko, V.V., Pis’menskaya, N.D., Belova, E.I., and Lopatkova, G.Yu., RF Patent No. 2008141949 (2008).
Sharafan, M.V. and Zabolotskii, V.I., RF Patent No. 78577 (2008).
Lopatkova, G.Yu., Cand. Sci. (Chem.) Dissertation, Krasnodar, 2006.
Zabolotskii, V.I., Shel’deshov, N.V., and Sharafan, M.V., Russ. J. Electrochem., 2006, vol. 42, p. 1345.
Zabolotskii, V.I., Sharafan, M.V., Shel’deshov, N.V., and Lovtsov, E.G., Russ. J. Electrochem., 2008, vol. 44, p. 141.
Zabolotskii, V.I., Sharafan, M.V., and Shel’deshov, N.V., Russ. J. Electrochem., 2008, vol. 44, p. 1127.
Sharafan, M.V., Zabolotskii, V.I., and Bugakov, V.V., Russ. J. Electrochem., 2009, vol. 45, p. 1162.
Levich, V.G., Fiziko-khimicheskaya gidrodinamika (Physicochemical Hydrodynamics), Moscow: Fizmatgiz, 1959.
Zabolotskii, V.I., Ganych, V.V., and Shel’deshov, N.V., Elektrokhimiya, 1991, vol. 27, p. 1245.
Chermit, R.H., Zabolotskii, V.I., Sharafan, M.V., and Bugakov, V.V., Ion Transport in Organic and Inorganic Membranes: Materials. Proceed. Intern. Conf., 2011.
Choi, J.-H., Moon, S.-H., J. Colloid Interface Sci., 2003, p. 93.
Bugakov, V.V., Zabolotskii, V.I., and Sharafan, M.V., Sorbtsionnye Khromatogr. Protsessy, 2010, vol. 10, p. 870.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.I. Zabolotskii, R.Kh. Chermit, M.V. Sharafan, 2014, published in Elektrokhimiya, 2014, Vol. 50, No. 1, pp. 45–52.
Rights and permissions
About this article
Cite this article
Zabolotskii, V.I., Chermit, R.K. & Sharafan, M.V. Mass transfer mechanism and chemical stability of strongly basic anion-exchange membranes under overlimiting current conditions. Russ J Electrochem 50, 38–45 (2014). https://doi.org/10.1134/S102319351401011X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S102319351401011X