Abstract
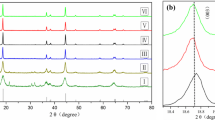
Cathode materials LiNi0.5Mn1.5O4 and LiNi0.5 − x/2La x Mn1.5 − x/2O4 (x = 0.04, 0.1, 0.14) were successfully prepared by the sol-gel self-combustion reaction (SCR) method. The X-ray diffraction (XRD) patterns indicated that, a few of doping La ions did not change the structure of LiNi0.5Mn1.5O4 material. The scanning electronic microscopy (SEM) showed that the sample heated at 800°C for 12 h and then annealed at 600°C for 10 h exhibited excellent geometry appearance. A novel electrolyte system, 0.7 mol L−1 lithium bis(oxalate)borate (LiBOB)-propylene carbonate (PC)/dimethyl carbonate (DMC) (1: 1, v/v), was used in the cycle performance test of the cell. The results showed that the cell with this novel electrolyte system performed better than the one with traditional electrolyte system, 1.0 mol L−1 LiPF6-ethylene carbonate (EC)/DMC (1: 1, v/v). And the electrochemical properties tests showed that LiNi0.45La0.1Mn1.45O4/Li cell performed better than LiNi0.5Mn1.5O4/Li cell at cycle performance, median voltage, and efficiency.
Similar content being viewed by others
References
Wronski, Z.S., Int. Mater. Rev., 2001, vol. 46, p. 1.
Shi, Z.C. and Yang, Y., Progress Chem., 2005, vol. 17, p. 604.
Armstrong, A.R. and Bruce, P.G., Electrochem. Solid State Lett., 2004, vol. 7, p. 1.
Li, S., Cheng, J.F., and Ji, S.J., Rare. Metal. Mat. Eng., 2003, vol. 32, p. 468.
Lee, Y.S. and Yoshio, M., Electrochem. Solid State Lett., 2001, vol. 4, p. 166.
Van, D.V.A., Marianetti, C., and Morgan, C., Solid State Ion., 2000, vol. 135, p. 21.
Idemoto, Y., Narai, H., and Koura, N., J. Power Sources, 2003, vol. 119–121, p. 125.
Kim, J.H., Myung, S.T., and Sun, Y.K., Electrochim. Acta, 2004, vol. 49, p. 219.
Wu, X. and Kim, S.B., J. Power Sources, 2002, vol. 109, p. 53.
Ooms, F.G.B., Kelder, E.M., and Schoonman, J., Solid State Ion., 2002, vols. 152–153, p. 143.
Rojas, R.M., Amarilla, J.M., Pascual, L., and Rojo, J.M., J. Power Sources, 2006, vol. 160, p. 529.
KiJoo, H. and Yang, K.S., J. Power Sources, 2002, vol. 109, p. 427.
Alcantara, R., Jaraba, M., and Lavela, P., Electrochim. Acta, 2002, vol. 47, p. 1829.
Sung, B.P., Won, S.E., and Ho, J., J. Power Sources, 2006, vol. 159, p. 679.
Yang, L., Furczon, M.M., and Xiao, A., J. Power Sources, 2010, vol. 195, p. 1698.
Xu, K., Zhang, S.S., and Jow, T.R., Electrochem. Solid State Lett., 2002, vol. 5, p. 26.
Panitz, J.C., Wietelmann, U., and Wachtler, M., J. Power Sources, 2006, vol. 153, p. 396.
Jiang, J. and Dahn, J.R., Electrochem Solid State Lett., 2003, vol. 6, p. 180.
Fan, W.F., Qu, M.Z., and Peng, G., Chinese J. lnorg. Chem., 2009, vol. 25, p. 124.
Fang, X., Lu, Y., and Ding, N., Electrochim. Acta, 2010, vol. 55, p. 832.
Zhong, Q.M., Bonakdarpour, A., and Zhang, M.J., Electrochem. Soc., 1997, vol. 144, p. 205.
Xi, X.R., Wang, S.C., and Zhan, Z.K., Min. Metall. Eng., 2009, vol. 29, p. 85.
Yan, Q.X., Wang, Z.X., and Wu, J., J. Funct. Mater., 2009, vol. 40, p. 933.
Nakai, I., Shiraishi, Y., and Nishikawa, F., Spectrochim. Acta, Part B, 1999, vol. 54, p. 143.
Meng, M.W., Liao, Q.H., and Huang, Y., Heat Treat. Met., 2009, vol. 34, no. 3, p. 10.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Elektrokhimiya, 2014, Vol. 50, No. 4, pp. 407–414.
The article is published in the original.
Rights and permissions
About this article
Cite this article
Cui, X., Shi, X., Li, G. et al. Electrochemical performance of LiNi0.5Mn1.5O4 doped with la and its compatiblity with new electrolyte system. Russ J Electrochem 50, 363–369 (2014). https://doi.org/10.1134/S102319351310011X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S102319351310011X