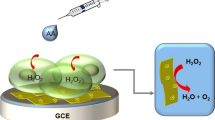
Abstract
In this Letter, we demonstrate for the first time that 7,7,8,8-tetracyanoquinodimethane microsheets (TCNQMSs) can effectively catalyze the reduction of H2O2 because TCNQ is a strong electron acceptor, TCNQMSs modified glass carbon electrode (GCE) is prepared by a simple casting method. This H2O2 sensor shows a fast amperometric response time of less than 3 s, and the corresponding linear range is estimated to be from 3 to 238 mM (r = 0.992) and a detection limit of 4.76 μM at a signal-to-noise ratio of 3. Our present study is important because it provides us a new structure for H2O2 detection application.
Similar content being viewed by others
References
Klassen, N., Marchington, D., and McGovan, H.C.E., Anal. Chem., 1994, vol. 66, p. 2921.
Chang, M.C.Y., Pralle, A., Isacoff, E.Y., and Chang, C.J., J. Am. Chem. Soc., 2004, vol. 126, p. 15392.
Wang, B., Zhang, J., Pan, Z., Tao, X., and Wang, H., Biosens. Bioelectron., 2009, vol. 24, p. 1141.
Song, Y., Wang, L., Ren, C., Zhu, G., and Li, Z., Sens. Actuators B, vol. 114, p. 1001.
Tanner, P.A. and Wong, A.Y.S., Anal. Chim. Acta, 1998, vol. 370, p. 279.
Dringen, R., Kussmaul, L., and Hampreeht, B., Brain Research Protocols, 1998, vol. 2, p. 223.
Carmine, T.C., Bruchelt, G., Hahn, T., and Niethammer, D., J. Biolumin. Chemilumin., 1994, vol. 9, p. 267.
Bui, M.N., Pham, X., Han, K.N., Li, C.A., Kim, Y.S., and Seong, G.H., Sen Actuators B, 2010, vol. 150, p. 436.
Welch, C.M., Banks, C.E., Simm, A.O., and Compton, R.G., Anal. Bioanal. Chem., 2005, vol. 382, p. 12.
Li, Y., Zhang, J., Xuan, J., Jiang, L., and Zhu, J., Electrochem. Commun., 2010, vol. 12, p. 777.
Tian, J., Liu, S., and Sun, X., Langmuir, 2010, vol. 26, p. 15112.
Liu, S., Tian, J., Wang, L., Li, H., Zhang, Y., and Sun, X., Macromolecules, 2010, vol. 43, p. 10078.
Tseng, T.C., Urban, C., Wang, Y., Otero, R., Tait, S.L., Alcami, M., Ecija, D., Trelka, M., Gallego, J.M., Lin, N., Konuma, M., Starke, U., Nefedov, A., Langner, A., Woll, C., Herrang, M.A., Martin, F., Kern, K., and Miranda, R., Nat. Chem, 2010, vol. 2, p. 374.
Chae, I.S., Kang, S.W., Park, J.Y., Lee, Y.G., Lee, J.H., Won, J., and Kang, Y.S., Angew. Chem. Int. Ed., 2011, vol. 50, p. 2982.
Heintz, R.A., Zhao, H.H., Xiang, O.Y., Grandinetti, G., Cowen, J., and Dunbar, K.R., Inorg. Chem., 1999, vol. 38, p. 144.
Miyasaka, H., Campos-Fernandez, C.S., Clerac, R., and Dunbar, K.R., Angew. Chem., Int. Ed. Engl., 2000, vol. 39, p. 3831.
Miyasaka, H., Motokawa, N., Matsunaga, S., Yamashita, M., Sugimoto, K., Mori, T., Toyota, N., and Dunbar, K.R., J. Am. Chem. Soc., 2010, vol. 132, p. 1532.
Shimomura, S., Yanai, N., Matsuda, R., and Kitagawa, S., Inorg. Chem., 2011, vol. 50, p. 172.
Li, H., Wang, L., Zhai, J., Luo, Y., Zhang, Y., Tian, J., and Sun, X., Anal. Methods, 2011, vol. 3, p. 1051.
Bard, A.J. and Faulkner, L.R., Electrochemical Methods: Fundamentals and Applications, N.Y.: Inc. John Wiley & Sons, 2001, 2nd ed.
Lin, D., Jiang, Y., Wang, Y., and Sun, S., J. Nanomater, 2008, vol. 1, p. 473791.
Lu, W., Liao, F., Luo, Y., Chang, G., and Sun, X., Electrochim. Acta, 2011, vol. 56, p. 2295.
Shabanov, O.M., Ismailova, F.O., Maksumova, D.G., Gadzhiev, S.M., and Magomedova, A.O., Russ. J. Electrochem., 2006, vol. 42, p. 986.
Fridman, V.A., Bogdanovskaya, V.A., and Tarasevich, M.R., Russ. J. Electrochem., 1998, vol. 34, p. 56.
Xu, X.X., Zhang, J.X., Guo, F., Zhang, W., Zhou, H.M., Wang, B.L., Zheng, Y.F., Wang, Y.B., Cheng, Y., Luo, X., and Jang, B.Z., Colloids Surf. B: Biointerfaces, 2011, vol. 84, p. 427.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Elektrokhimiya, 2013, Vol. 49, No. 11, pp. 1219–1222.
The article is published in the original.
Rights and permissions
About this article
Cite this article
Qin, X., Lu, W., Luo, Y. et al. 7,7,8,8-tetracyanoquinodimethane microsheets for hydrogen peroxide reduction. Russ J Electrochem 49, 1097–1100 (2013). https://doi.org/10.1134/S1023193512080071
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193512080071