Abstract
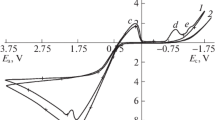
The regularities of silver anodic dissolution are studied by using the voltammetry (at the potential scan rates from 5 to 1000 mV/s) on the electrode, which was renewed immediately in the solution by cutting-off a thin surface metal layer, and quartz microgravimetry, for various concentrations of sodium thiosulfate (0.05–0.2 M). It is shown that, in the potential range from 0 to 0.4 V (normal hydrogen electrode), the polarization curves reflect the silver dissolution, whereas the contribution of oxidation of S2O 2−3 ions is insignificant. At low potential scan rates, the process kinetics is of mixed nature. The kinetics and mechanism of anodic process are studied by using the measurements at high potential scan rates (100–200 mV/s) and the calculations of equilibrium composition of near-electrode layer. It is found that the exchange current in the electrolytes studied is 5 × 10−5 A/cm2, the transfer coefficient α is approximately 0.5, and both parameters are virtually independent of the concentration of S2O 2−3 ions. The reaction order of silver dissolution with respect to the ligand \( \left. {\frac{{\partial logi}} {{\partial logc}}} \right|_E \) is close to unity and is independent of potential. With regard for the literature data on the adsorption of thiosulfate ions on silver, this result is interpreted as the evidence for the involvement of one S2O 2−3 ion from bulk solution, along with adsorbed ligands, in the elementary act of metal dissolution.
Similar content being viewed by others
References
Mineev, G.G. and Panchenko, A.F., Rastvoriteli zolota i serebra v gidrometallurgii (Solvents of Gold and Silver in Hydrometallurgy), Moscow: Metallurgiya, 1994.
Jeffrey, M.J., Hydrometallurgy, 2001, vol. 60, p. 7.
Mineev, G.G., Zhuchkov, I.A., Punishko, O.A., Mineeva, T.S., and Aksenov, A.V., Izv. Vyssh. Uchebn. Zaved. RF: Tsvet. Metallurgiya, 2005, no. 2, p. 8.
Meretukov, M.A., Tsvet. Metally, 2005, nos. 5–6, p. 105.
Breuer, P.L., Jeffrey, M.J., Tan, E.H.K., and Bott, A.W., J. Appl. Electrochem., 2002, vol. 32, p. 1167.
Hubin, A. and Vereecken, J., J. Appl. Electrochem., 1994, vol. 24, p. 239.
Red’ko, A., Osnovy cherno-belykh i tsvetnykh fotoprotsessov (Fundamentals of Black-White and Colored Photoprocesses), Moscow: Iskusstvo, 1990.
Bek, R.Yu., Rogozhnikov, N.A., and Kosolapov, G.V., Elektrokhimiya, 1997, vol. 33, p. 131 [Russ. J. Electrochem. (Engl. Transl.), vol. 33, p. 119].
Bek, R.Yu. and Rogozhnikov, N.A., Elektrokhimiya, 1997. T. 33. P. 629 [Russ. J. Electrochem., vol. 33, p. 579].
Zelinskii, A.G. and Bek, R.Yu., Elektrokhimiya, 1985, vol. 21, p. 66.
Aleksandrova, T.P., Ovchinnikova, S.N., Vais, A.A., and Bek, R.Yu., Zh. Anal. Khim., 1999, vol. 54, p. 732 [J. Anal. Chem. (Engl. Transl.), vol. 54, p. 646].
Sauerbrey, G., Z. Phys., 1959, vol. 155, p. 206.
Vatankhah, G., Lessard, J., Jerkiewicz, G., Zolfaghari, A., and Conway, B.E., Electrochim. Acta, 2003, vol. 48, p. 1613.
Spravochnik po elektrokhimii (Handbook on Electrochemistry), Sukhotin, A.M., Ed., Leningrad: Khimiya, 1981.
Galus, Z., Teoretyczne podstawy electroanalizy chemicznej, Warszawa: Panstwowe Wydawnictwo Naukowe, 1971.
Bek, R.Yu., Shuraeva, L.I., Ovchinnikova, S.N., and Kenzin, V.I., Elektrokhimiya, 2007, vol. 43, p. 1329 [Russ. J. Electrochem., vol. 43, p. 1260].
Pyatnitskii, I.V. and Sukhan, V.V., Analiticheskaya khimiya serebra (Analytical Chemistry of Silver), Moscow: Nauka, 1975.
Bjerrum, J., Stability Constants. Pt II. Inorganic Ligands, London: Chem. Soc, 1958.
Smith, R. and Martell, A., Critical Stability Constants. V. 4. Inorg. Compl, New York: Plenum, 1976, p. 256.
Kubach, J., Gmelins Handbuch Der Anorgan Chem, Berlin: Springer, 1975, Ag [B3].
Hubin, A. and Vereecken, J., J. Appl. Electrochem., 1994, vol. 24, p. 396.
Vandeputte, S., Verboom, E., Hubin, A., and Verecken, J., J. Electroanal. Chem., 1995, vol. 397, p. 249.
Block, P.A. and Stevens, G.W., J. Photogr. Sci., 1967, vol. 9, p. 330.
Ramstad, T. and Milner, D., Anal. Instrum., 1989, vol. 18, p. 147.
Srinivasan, R. and Suni, J., J. Appl. Electrochem., 1998, vol. 28, p. 993.
Bek, R.Yu. and Rogozhnikov, N.A., Elektrokhimiya, 1995, vol. 31, p. 1121 [Russ. J. Electrochem., vol. 31, p. 1121].
Bek, R.Yu. and Shuraeva, L.I., Elektrokhimiya, 1997, vol. 33, p. 636 [Russ. J. Electrochem., vol. 33, p. 586].
Bek, R.Yu. and Shevtsova, O.N., Elektrokhimiya, 2010, vol. 46, p. 1052 [Russ. J. Electrochem., vol. 46, p. 987].
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © R.Yu. Bek, O.N. Shevtsova, 2011, published in Elektrokhimiya, 2011, Vol. 47, No. 3, pp. 267–274.
Rights and permissions
About this article
Cite this article
Bek, R.Y., Shevtsova, O.N. Kinetics of silver anodic dissolution in thiosulfate electrolytes. Russ J Electrochem 47, 248–255 (2011). https://doi.org/10.1134/S1023193511030037
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193511030037