Abstract
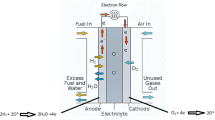
The high-temperature synthesis based on commercial catalyst E-TEK (40% Pt) using cobalt, chromium, and iron organic precursors as well as d-metal salts yielded PtM (1:1) catalysts (PtCo, PtCr, PtMn, PtNi, PtFe, and PtV) for electroreduction of molecular oxygen in concentrated H3PO4 at the temperature of 160°C. The phase composition of the synthesized catalysts was studied by powder diffraction. The electrochemical measurements were carried out in 15 M H3PO4 at 20 and 160°C using a model gas diffusion electrode. An assumption was made that close charging curves recorded for synthesized PtM catalysts in both hydrogen and oxygen adsorption ranges were due to formation of the core-shell structure: alloy core and surface layers enriched with platinum. The Tafel curves of molecular oxygen reduction in 15 M H3PO4 at 160°C were characterized with the sole slope of 0.10 to 0.11 V. The catalytic activity in the range of potentials from 0.8 to 0.9 V (RHE) was shown approximately twice as that of pure platinum catalyst. The highest activity was recorded for PtCo and PtCr binary catalysts. Their use in middle-temperature hydrogen-air fuel cells with solid polymeric electrolyte based on polybenzimidazole doped with phosphoric acid enabled 2- to 3-fold decrease of the platinum share in the cathode.
Similar content being viewed by others
References
Qinfeng, Li., Ronghuan, He., Gao, J.A., Jensen, J.O., and Bjerrum, N.J., J. Electrochem. Soc., 2003, vol. 150.
Pereira, L.G.S., Paganin, V.A., and Ticianelli, E.A., Electrochim. Acta, 2009, vol. 54, p. 1992.
Zhenyu, Liu, Wainright, J.S., Litt, M.H., and Savinell, R.F., Electrochim. Acta, 2006, vol. 51, p. 3914.
Landsmann, D.A. and Luczak, F.J., Handbook of Fuel Cells, 2003, vol. 4.
Wang, J.T., Savinell, R.F., Wainringht, J.S., Litt, M.H., and Yu, H., Electrochim. Acta, 1996, vol. 41, p. 193.
Modestov, A.D., Tarasevich, M.R., Leikin, A.Yu., Filimonov, V.Ya., and Zagudaeva, N.M., Al’Ternativnaya Energetika I Ekologiya, 2008, no. 10, p. 83.
Tarasevich, M.R., Lubnin, E.N., Zagudaeva, N.M., and Maleeva, E.A., Korroziya: Materialy, Zashchita, 2007, no. 10, p. 15.
Stonehard, P., J. Appl. Electrochem., 1992, vol. 22, p. 995.
Thompsett, D., Handbook of Fuel Cells, 2003, vol. 3.
Jalan, V.M., US Pat. 507907, 1992.
Zagudaeva, N.M., Tarasevich, M.R., and Maleeva, E.A., Al’Ternativnaya Energetika I Ekologiya, 2007, no. 2, p. 110.
Breault, R.D., Handbook of Fuel Cells, 2003, vol. 4.
Bogdanovskaya, V.A., Tarasevich, M.R., Kuznetsova, L.N., and Radina, M.V., Zh. Fiz. Khim., 2009, vol. 83, p. 2244 [Russ. J. Phys. Chem. (Engl. Transl.), vol. 83, p. ].
Zagudaeva, N.M., Tarasevich, M.R., and Maleeva, E.A., Al’Ternativnaya Energetika I Ekologiya, 2007, no. 8, p. 79.
Landsmann, D.A. and Luczak, F.J.., US Pat. 4 316 944, 1982.
Antolini, E., Salgado, Jose R.C., and Gonzalez, E., J. Power Sources, 2006, vol. 160, p. 957.
Nilekar, A.U., Xu, Y., Zhang, J., Vukmirovic, M.B., Sasaki, K., Adzic, R., and Mavrikakis, M., Top. Catalysis, 2007, vol. 46, p. 276.
Kitchin, J.R., Norskov, J.K., Barteau, M.A., and Chen, J.G., J. Chem. Phys., 2004, vol. 120, p. 10240.
Goreeley, J., Norskov, J.K., and Mavrikakis, M., Ann. Rev. Phys. Chem., 2002, vol. 53, p. 319.
Tarasevich, M.R., Elektrokhimiya, 1973, vol. 9, p. 578.
Tarasevich, M.R., Sadkovsky, A., and Yeager, E., Comprehensive Treatise of Electrochemistry, New York: Plenum, 1982, vol. 7, p. 301.
Damjanovic, A., Genshaw, M.A., and Bockris, J.O.M., J. Chem. Phys., 1966, vol. 45, p. 4057.
Huang, J.C., Sen, R.K., and Yeager, E., J. Electroanal. Chem., 1979, vol. 126, p. 786.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © N.M. Zagudaeva, M.R. Tarasevich, 2010, published in Elektrokhimiya, 2010, Vol. 46, No. 5, pp. 562–568.
Rights and permissions
About this article
Cite this article
Zagudaeva, N.M., Tarasevich, M.R. Electrochemical characteristics of platinum-based binary catalysts for middle-temperature hydrogen-air fuel cells with phosphoric acid electrolyte. Russ J Electrochem 46, 530–536 (2010). https://doi.org/10.1134/S102319351005006X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S102319351005006X