Abstract
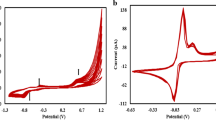
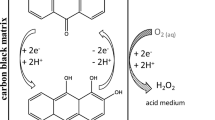
In this paper, the powder microelectrode technique was employed in studying the voltametric response of the O2/ −2 couple, which demonstrated a nearly reversible redox process at an acetylene black powder microelectrode in N,N-dimethylformamide (DMF). A well-developed steady state current plateau for the electrochemical reduction of oxygen was obtained in this system. The electron transfer number (n) and heterogeneous electron transfer rate constant k s were measured by steady-state voltametric response, and the results were 1.08, 3.4 × 10−3 cm s−1, respectively. Additionally, the scavenging activity of O −2 with biological antioxidant (ascorbic acid) was evaluated by cyclic voltammetry; IC50 came to 5 × 10−4 mol/l.
Similar content being viewed by others
References
Nekrasov, L.N., Dukhanova, L.M., Dubrovina, N.I., and Vykhodtseva, L.N., Elektrokhimiya, 1970, vol. 6, p. 388.
Nekrasov, L.N. and Dubrovina, N.I., Elektrokhimiya, 1972, vol. 8, p. 946.
Wei, Y.L., Xiao, X.B., Dang, X.P., and Hu, S.S., Bioelectrochemistry, 2003, vol. 61, p. 51.
Peressini, S., Tavagnacco, C., Costa, G., and Amatore, C., J. Electroanal. Chem., 2002, vol. 532, p. 295.
Ortiz, M.E., Núñez-Vergara, L.J., and Squella, J.A., J. Electroanal. Chem., 2002, vol. 519, p. 46.
Ortiz, M.E., Núñez-Vergara, L.J., and Squella, J.A., J. Electroanal. Chem., 2003, vol. 549, p.157.
Rotenberg, Z.A., Alpatova, N.M., Ovsyannikova, E.V., Kirchmeyer, S.S., and Jonas, F., Russ. J. Electrochem., 2002, vol. 38, p. 1244.
Cha, C.S., Li, C.M., Yang, H.X., and Liu, P.F., J. Electroanal. Chem., 1994, vol. 368, p. 47.
Chen, J. and Cha, C.S., J. Electroanal. Chem., 1999, vol. 463, p. 93.
Liu, P.F., Lu, J.T., and Yan, J.W., J. Electroanal. Chem., 1999, vol. 469, p. 196.
Tu, W.Y., Liu, W.J., Cha, C.S., and Wu, B.L., Electrochim Acta, 1998, vol. 43, p. 3731.
Sawyer, D.T., Chlercalo, G., Jr., Angells, C.T., Nanni, E.J. Jr., and Tsuchlya, T., Anal. Chem., 1982, vol. 54, p. 1720.
Galus, Z., Golas J., and Osteryoung, J., J. Phys. Chem., 1988, vol. 92, p. 1103.
Tsushima, M., Tokuda, K., and Ohsaka, T., Anal. Chem., 1994, vol. 66, p. 4551.
Wang, B. and Cao, X., J. Electroanal. Chem., 1991, vol. 309, p. 147.
Meister, A., J. Biol. Chem., 1994, vol. 269, p. 9397.
Gao, R.M., Yuan, Z.B., and Zhao, Z.Q., Bioelectrochem. Bioenerg., 1998, vol. 45, p. 41.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Elektrokhimiya, 2008, Vol. 44, No. 8, pp. 1040–1044.
The text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Wei, Y., Zhang, S. Study on the electroreduction process of oxygen to superoxide ion by using acetylene black powder microelectrode. Russ J Electrochem 44, 967–971 (2008). https://doi.org/10.1134/S1023193508080144
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193508080144