Abstract
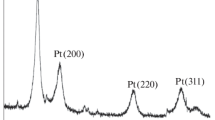
The composite coating Pt-MoOx is produced by an electrochemical technique under potentiodynamic conditions on the surface of a preliminarily prepared electrode of glassy carbon. The inclusion of molybdenum into the composition of the obtained electrode deposit is confirmed by the data of cyclic voltammetry and the secondary-electron emission spectra. In the cyclic voltammograms that are obtained in a 2 M solution of sulfuric acid one can distinguish a pair of peaks at potentials equal to 0.46 V (anodic run) and 0.3 V (cathodic run), which are connected with the redox transitions experienced by molybdenum compounds. It is discovered that the obtained deposit possesses catalytic properties with respect to the oxygen reduction reaction. The number of electrons that are corresponding to the redox transitions experienced by molybdenum compounds is calculated. It amounts to 0.27 electrons per molybdenum atom.
Similar content being viewed by others
References
Fournier, J., Faubert, G., Tilguin, J.Y., Cote, R., Guay, D., and Dodelet, J.P., Electrochem. Soc., 1997, vol. 144, p. 145.
Tamizhmani, G. and Capuano, G., J. Electrochem. Soc., 1994, vol. 141, p. 968.
Swette, L. and Kackley, N., J. Power Sources, 1990, vol. 29, p. 423.
Wang, H.-S., Acta Chim. Sin., 2002, vol. 60, p. 606.
Kosminsky, L. and Bertotti, M., J. Electroanal. Chem., 1999, vol. 471, p. 37.
Wang, Y., Fachini, E., Cruz, G., Zhu, Y., Ishikawa, Y., Colucci, J.A., and Cabrera, C.R., J. Electrochem. Soc., 2001, vol. 148, p. 222.
Chepeleva, S.A., Extended Abstract of Cand. Sci. (Chem.) Dissertation, Moscow: RKhTU, 2005.
Monk, P.M.S., Ali, T., and Partridge R.D., Solid State Ionics, 1995, vol. 80, p. 75.
Shen, K.J. and Tseung, A.C.C., J. Electrochem. Soc., 1996, vol. 43, p. 2703.
Kulesza, P.J. and Faulkner, L.R., J. Electroanal. Chem., 1989, vol. 259, p. 81.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.V. Kuznetsov, A.A. Kalinkina, T.V. Pshenichkina, 2007, published in Elektrokhimiya, 2007, Vol. 43, No. 7, pp. 815–821.
Rights and permissions
About this article
Cite this article
Kuznetsov, V.V., Kalinkina, A.A. & Pshenichkina, T.V. Electrochemical properties of composite materials based on platinum modified with molybdenum compounds. Russ J Electrochem 43, 776–781 (2007). https://doi.org/10.1134/S1023193507070063
Received:
Issue Date:
DOI: https://doi.org/10.1134/S1023193507070063